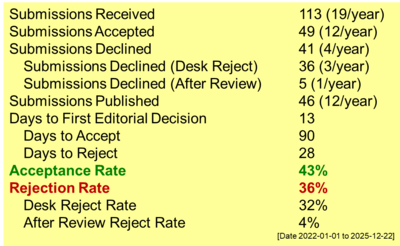
Carbon Paste Electrode Modified Zeolite-Iron as a Chromium(VI) Detection Medium
DOI:
https://doi.org/10.55749/ijcs.v3i2.64Keywords:
Carbon paste electrode, Chromium(VI), Cyclic voltammetry, Iron-modified zeoliteAbstract
Chromium exists in two dominant species in nature, Cr(III) and Cr(VI). Both are stable; however, Cr(VI) exhibits significantly higher toxicity than Cr(III). Existing measurement methods could not differentiate between these two chromium species. Therefore, a more sensitive and selective measurement method was required for their speciation, particularly for Cr(VI) detection. In this study, a carbon paste electrode modified with zeolite and iron was developed for Cr(VI) measurement using the voltammetry method. The electrode was prepared by mixing graphite, iron-modified zeolite, and liquid paraffin. Measurements were conducted using cyclic voltammetry within a potential range of -1.2 V to 1.2 V. A 0.05 M KCl solution was used as the electrolyte. Electrode characterization was carried out concerning three parameters: the effect of analyte pH, preconcentration time, and the composition of iron-modified zeolite. The optimum Cr(VI) measurement was achieved in a 50 μM Cr(VI) solution at pH 3 using a carbon paste electrode with 20% iron-modified zeolite and a preconcentration time of 25 min. Under these optimal conditions, a cathodic peak current of 5.22 μA was obtained.
References
Swaroop A., Bagchi M., Preuss H.G., Stone S.Z., Ahmad T., & Bagchi D. 2019. Benefits of Chromium(III) Complexes in Animal and Human Health. In The nutritional biochemistry of chromium. pp. 251-278. doi: https://doi.org/10.1016/B978-0-444-64121-2.00008-8.
Pratiwi D.Y. 2020. Dampak pencemaran logam berat (timbal, tembaga, merkuri, kadmium, krom) terhadap organisme perairan dan kesehatan manusia. J. Akuatek. 1(1). 59–65. doi: https://doi.org/10.24198/akuatek.v1i1.28135.
Jobby R., Jha P., Yadav A.K., & Desai N., 2018 Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere. 207. 255-266. doi: https://doi.org/10.1016/j.chemosphere.2018.05.050.
Arif Z., Munandar R., Rohaeti E., & Rafi M. 2023. Detection of hexavalent chromium ion water by optode membrane. Indones J Chem Stud. 2(2). 76–82. doi: https://doi.org/10.22749/ijcs.v212.39.
Kumar N., Madwhal D., Jain V.K., Suman. 2020. A POC device for on-the-spot detection of hexavalent chromium in wastewater. J. Environ. Chem. Eng. 8(5). 104342. doi: https://doi.org/10.1016/j.jece.2020.104342.
Arif Z., Fendy, Ananjaya A.A., Saprudin D., Rohaeti E. 2024. Simple synthesis of cellulose triacetate from hvs paper waste and its application for optode. Indones. J. Chem. Stud. 3(1). 16-21. doi: https://doi.org/10.55749/ijcs.v3i1.46.
Dvoynenko O., Lo S., Chen Y., Chen G.W., Tsai H., Wang Y., Wang J. 2021. Speciation analysis of Cr(VI) and Cr(III) in water with surface-enhanced raman spectroscopy. ACS Omega. 6(3). 2052-2059. doi: https://doi.org/10.1021/acsomega.0c05020.
Herlina H., Zulfikar M.A., & Buchari B. 2018. Cyclic voltammetry study of mediated electrochemical oxidation using platinum wire, Pt/Co(OH)2 and Pt/Co electrodes in various supporting electrolytes. JKPK. 3(2). 82–92. doi: https://doi.org/10.20961/jkpk.v3i2.22330.
Thatikayala D. Noori M.T. Min B. 2023. Zeolite-modified electrodes for electrochemical sensing of heavy metal ions–Progress and future directions. Mater. Today Chem. 29. 101412. doi: https://doi.org/10.1016/j.mtchem.2023.101412.
Suhartana, Sukmasari E., Azmiyawati C. 2018. Modification of natural zeolite with Fe(III) and its application as adsorbent Chloride and Carbonate ions. IOP Conf. Ser. Mater. Sci. Eng. 349(1). 012075. doi: https://doi.org/10.1088/1757-899X/349/1/012075.
Al-Ani A., Drijfhout F., Cricket S., & Zholobenko V. 2019. Catalytic performance of microporous materials fot the production of renewable fuels. J. Porous Mater. 26(1). 69–76. doi: https://doi.org/10.1007/s10934-018-0610-7.
Oktaviani R., Hindryawati N., & Panggabean A.S. 2019. Modifikasi dan karakterisasi zeolit alam tasikmalaya dengan Fe2O3. Jurnal Atomik. 4(1). 30–35.
Ngapa Y.D., & Ika Y.E. 2020. Adsorpsi pewarna biru metilena dan jingga metil menggunakan adsorben zeolit alam ende-Nusa Tenggara Timur (NTT). Indo. J. Chem. Res. 8(2). 151–158. doi: https://doi.org/10.30598/ijcr.2020.8-ydn.
Sutopo FXR. 1991. Pengkajian karakteristik zeolit Tasikmalaya dan pemanfaatan dalam pengolahan air. Bandung: Pusat Penelitian dan Pengembangan Teknologi Mineral dan Batubara.
Wyantuti S. 2008. Karakterisasi Zeolit Alam Asal Tasikmalaya. Bandung: Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Padjajaran.
Las T. 2005. Potensi Zeolit untuk Mengolah Limbah Industri dan Radioaktif. Tangerang: Pusat Teknologi Limbah Radioaktif-Badan Tenaga Nuklir Nasional (PTLR-BATAN), Kawasan Puspiptek Serpong.
Lang Q., Lu P., Yang X., & Valtchev V. 2024. Zeolites for the environment. Green Carbon. 2(1). 12–32. doi: https://doi.org/10.1016/j.greenca.2024.02.007.
Pardi H., & Willian N. 2022. Penentuan logam Cr(VI) menggunakan metoda voltammetri stripping anoda (VSA) pada sampel air laut dan air sungai. Al-Kimia, 10(1). 32–41. doi: https://doi.org/10.24252/al-kimia.v10i1.21799.
Ray A., Mukhopadhyay I., & Pati K.R. 2018. Electrocatalysts for Fuel Cells and Hydrogen Evolution-Theory to Design. IntechOpen. doi: https://doi.org/10.5772/intechopen.72563.
Gea S., Irvan I., Wijaya K., Nadia A., Pulungan A.N., Sihombing J.L., & Rahayu R. 2022. Bio-oil hydrodeoxygenation over acid activated-zeolite with different Si/Al ratio. Biofuel Res. J. 9(2). 1630-1639. doi: https://doi.org/10.18331/BRJ2022.9.2.4.
Li Y., Wu, Y., Puranik S., Lei Y., Vadas, T., & Li B. 2014. Metals as electron acceptors in single-chamber microbial fuel cells. J. Power Sources. 269. 430–439. doi: https://doi.org/10.1016/j.jpowsour.2014.06.117.
Saraswati T.E., Bahrudin A., & Anwar M. 2016. Pengaruh suhu pemanasan dan agen pengikat dalam pembuatan konduktor listrik berbasis arang. ALCHEMY. 12(2). 167–178. doi: https://doi.org/10.20961/alchemy.v12i2.708.
Yulianto E. 2014. Pembuatan elektroda pasta karbon termodifikasi kitosan untuk analisis logam Cr(VI) dengan ion pengganggu Fe(II) dan Zn(II) secara cyclic stripping voltammetry. Unesa J. Chem. 3(3). 60–73. doi: https://doi.org/10.26740/ujc.v3n3.p%25p.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.