Magnetically Chitosan-Silica-Based Biosorbent as Efficient Removal of Au(III) in Artificial Wastewater
DOI:
https://doi.org/10.55749/ijcs.v3i1.40Keywords:
Adsorption, Gold(III) adsorption, Iron oxide nanoparticles, Silica-chitosan coating, Sol-gel synthesisAbstract
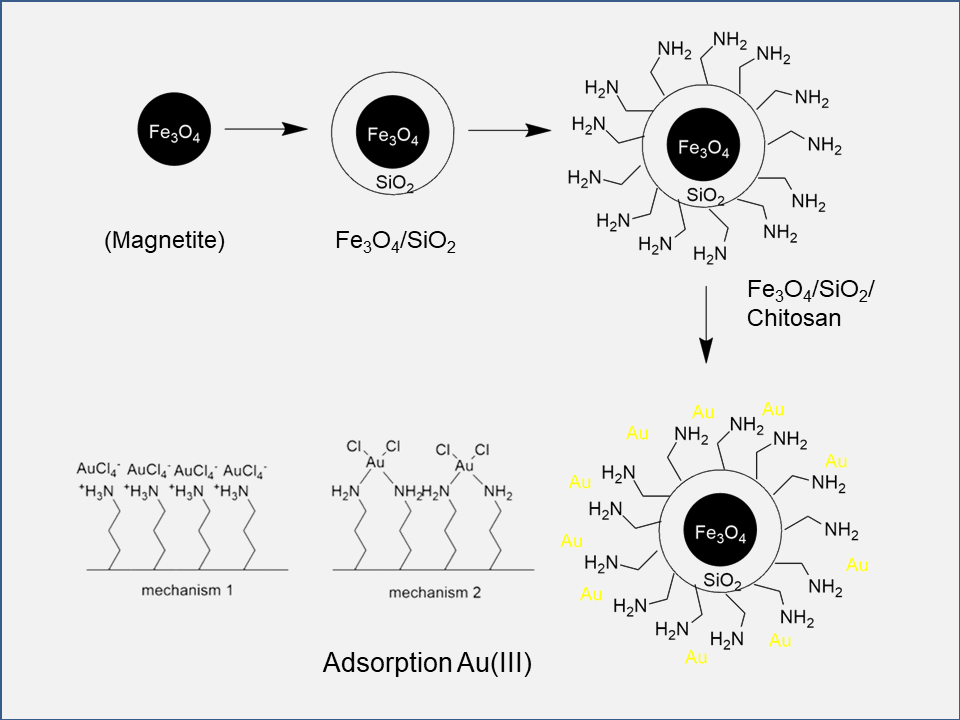
The synthesis of chitosan-modified silica-coated iron oxide magnetic material (Fe3O4/SiO2/Chitosan) via the sol-gel process addresses the need for enhanced stability and functionality in various applications. Coating iron oxide magnetic material with chitosan-modified silica is a common strategy to improve biocompatibility and performance. This study investigates the synthesis of Fe3O4/SiO2/Chitosan using sodium silicate as the silica precursor. The synthesis involved sonication of Fe3O4 and sodium silicate for 5 min, followed by adding chitosan in 4% acetic acid with continuous stirring. The mass ratio of Fe3O4:SiO2 was fixed at 0.5:0.73, with varying chitosan masses (0.025, 0.050, and 0.075 g). Characterization techniques used included Fourier-Transform Infrared Spectroscopy (FTIR), X-ray powder Diffraction (XRD), and Thermogravimetric analysis (TGA). The product with the highest mass yield was further analyzed. The variation in the amount of chitosan in the conducted research aimed to determine the optimum chitosan mass that could still bind to the silica framework. Magnetite was confirmed as the primary composition, with the addition of chitosan and silica functional groups observed through vibration absorption characteristics. Thermogravimetric analysis showed differences in decomposition patterns between samples. The optimal chitosan content for characterization was determined at 0.050 g. Future applications might include enhanced adsorption processes owing to the optimized structure and composition of Fe3O4/SiO2/Chitosan nanoparticles.
References
Minnaar, A. 2020. Water Pollution and Contamination from Gold Mines: Acid Mine Drainage in Gauteng Province, South Africa in In: Eman, K., Meško, G., Segato, L., Migliorini, M. (eds) Water, Governance, and Crime Issues. Springer, Cham. pp. 193–219. doi: https://doi.org/10.1007/978-3-030-44798-4_12
Carolin, C.F., Kumar, P.S., Saravanan, A., Joshiba, G.J., & Naushad, M. 2017. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 5(3). 2782–2799. doi: https://doi.org/10.1016/j.jece.2017.05.029.
Vidu, R., Matei, E., Predescu, A.M., Alhalaili, B., Pantilimon, C., Tarcea, C., & Predescu, C. 2020. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics. 8(4). 101. doi: https://doi.org/10.3390/toxics8040101.
Acheampong, M.A., Meulepas, R.J.W., & Lens, P.N.L. 2010. Removal of heavy metals and cyanide from gold mine wastewater. J. Chem. Technol. & Biotechnol. 85(5). 590–613. doi: https://doi.org/10.1002/jctb.2358.
Murakami, H., Nishihama, S., & Yoshizuka, K. 2015. Separation and recovery of gold from waste LED using ion exchange method. Hydrometallurgy. 157. 194–198. doi: https://doi.org/10.1016/j.hydromet.2015.08.014.
Gupta, A. et al. 2021. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials (Basel). 14(16). 4702. doi: https://doi.org/10.3390/ma14164702.
Wang, L.K., Wang, M.-H.S., Shammas, N.K., & Hahn, H.H. 2021. Physicochemical Treatment Consisting of Chemical Coagulation, Precipitation, Sedimentation, and Flotation BT - Integrated Natural Resources Research in Wang, L.K., Wang, M-H. S., and Hung, Y-T. Eds. Cham: Springer International Publishing, 265–397. doi: https://doi.org/10.1007/978-3-030-61002-9_6.
Sun, Y., Zhou, S., Chiang, P.-C., & Shah, K.J. 2019. Evaluation and optimization of enhanced coagulation process: Water and energy nexus. Water-Energy Nexus. 2(1). 25–36. doi: https://doi.org/10.1016/j.wen.2020.01.001.
Stenina, I., Golubenko, D., Nikonenko, V., & Yaroslavtsev, A. 2020. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 21(15). 5517. doi: https://doi.org/10.3390/ijms21155517.
Karamikamkar, S., Naguib, H.E., & Park, C.B. 2020. Advances in precursor system for silica-based aerogel production toward improved mechanical properties, customized morphology, and multifunctionality: A review. Adv. Colloid Interface Sci. 276. 102101. doi: https://doi.org/10.1016/j.cis.2020.102101.
Nowicki, W. 2019. Structural studies of complexation of Cu(II) with aminosilane-modified silica surface in heterogeneous system in a wide range of pH. Appl. Surf. Sci. 469. 566–572. doi: https://doi.org/10.1016/j.apsusc.2018.11.066.
Ngouangna, E.N., Manan, M.A., Oseh, J.O., Norddin, M.N.A.M., Agi, A., & Gbadamosi, A.O. 2020. Influence of (3–Aminopropyl) triethoxysilane on silica nanoparticle for enhanced oil recovery. J. Mol. Liq. 315. 113740. doi: https://doi.org/10.1016/j.molliq.2020.113740.
Dufil, Y., Gadenne, V., Carrière, P., Nunzi, J.-M., & Patrone, L. 2020. Growth and organization of (3-Trimethoxysilylpropyl) diethylenetriamine within reactive amino-terminated self-assembled monolayer on silica. Appl. Surf. Sci. 508. 145210. doi: https://doi.org/10.1016/j.apsusc.2019.145210.
Pradipta, A.R. & Irunsah, A. 2022. Synthesis of Modified TiO₂ Nanocomposite using Fe₃O₄ and Nickel as Photocatalyst in Reduction of Silver Ions. Indones. J. Chem. Stud. 1(1). 8–12. doi: https://doi.org/10.55749/ijcs.v1i1.7.
Pradipta, A.R., Mauludi, K., Kartini, I., & Kunarti, E.S. 2020. Synthesis of Fe3O4/TiO2 Nanocomposite as Photocatalyst in Photoreduction Reaction of CO2 Conversion to Methanol, in Symposium of Materials Science and Chemistry II, 2020, 840, 454–458. doi: https://doi.org/10.4028/www.scientific.net/KEM.840.454.
Putro, W.S., Lee, V.Y., Sato, K., Choi, J.-C., & Fukaya, N. 2021. From SiO2 to Alkoxysilanes for the Synthesis of Useful Chemicals. ACS Omega. 6(51). 35186–35195. doi: https://doi.org/10.1021/acsomega.1c05138.
Ebisike, K., Okoronkwo, A.E., & Alaneme, K.K. 2019. Adsorption of Cd (II) on chitosan–silica hybrid aerogel from aqueous solution. Environ. Technol. Innov. 14. 100337. doi: https://doi.org/10.1016/j.eti.2019.100337.
Sánchez Delgado, G.Y., do C Ferreira, F.H., Paschoal, D.F.S., & Dos Santos, H.F. 2022. The role of tridentate ligands on the redox stability of anticancer gold(III) complexes. J. Inorg. Biochem. 236. 111970. doi: https://doi.org/10.1016/j.jinorgbio.2022.111970.
Qiao, C., Ma, X., Wang, X., & Liu, L. 2021. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT. 135. 109984. doi: https://doi.org/10.1016/j.lwt.2020.109984.
Sugie, C., Navrotsky, A., Lauterbach, S., Kleebe, H.-J., & Mera, G. 2021. Structure and Thermodynamics of Silicon Oxycarbide Polymer-Derived Ceramics with and without Mixed-Bonding. Materials (Basel). 14(15). 4075. doi: https://doi.org/10.3390/ma14154075.
Shao, R., Niu, J., Zhu, F., Dou, M., Zhang, Z., & Wang, F. 2019. A facile and versatile strategy towards high-performance Si anodes for Li-ion capacitors: Concomitant conductive network construction and dual-interfacial engineering. Nano Energy. 63. 103824. doi: https://doi.org/10.1016/j.nanoen.2019.06.020.
Yao, M.Z., Liu, Y., Qin, C.N., Meng, X.J., Cheng, B.X., Zhao, H., Wang, S.F., & Huang, Z.Q. 2021. Facile fabrication of hydrophobic cellulose-based organic/inorganic nanomaterial modified with POSS by plasma treatment. Carbohydr. Polym. 253. 117193. doi: https://doi.org/10.1016/j.carbpol.2020.117193.
Gierszewska, M., Jakubowska, E., & Olewnik-Kruszkowska, E. 2019. Effect of chemical crosslinking on properties of chitosan-montmorillonite composites. Polym. Test. 77. 105872. doi: https://doi.org/10.1016/j.polymertesting.2019.04.019.
Karasyova, O., Ivanova, L., Lakshtanov, L., Lövgren, L., & Sjöberg, S. 1998. Complexation of Gold(III)-Chloride at the Surface of Hematite. Aquat. Geochemistry. 4. 215–231. doi: https://doi.org/10.1023/A:1009622915376.
Xi, W. & Haes, A.J. 2019. Elucidation of HEPES Affinity to and Structure on Gold Nanostars. J. Am. Chem. Soc. 141(9). 4034–4042. doi: https://doi.org/10.1021/jacs.8b13211.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.