Chemical Regeneration of Activated Carbon After Adsorption of Ni(II) Ions
DOI:
https://doi.org/10.55749/ijcs.v2i1.20Keywords:
Activated acarbon, Chemical regeneration, Ni(II) ionsAbstract
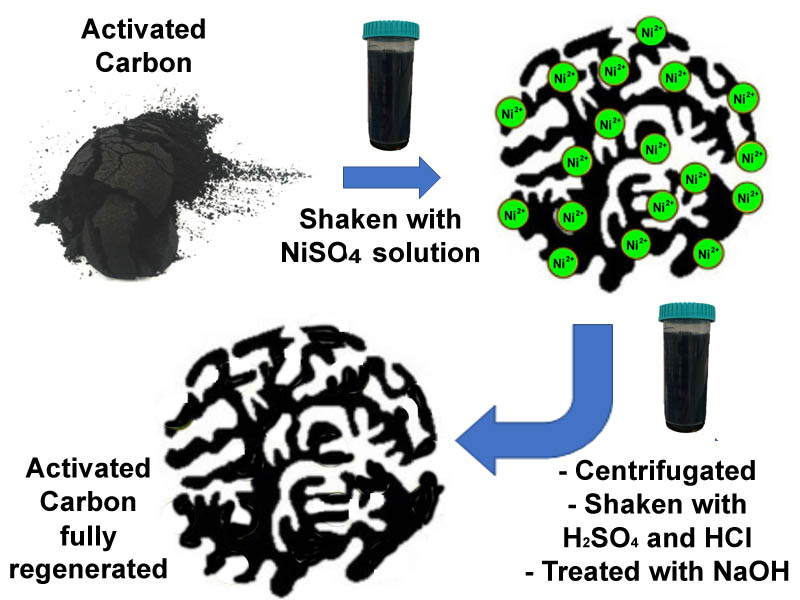
The chemical regeneration of activated carbon (AC) has recently received greater attention because it allows high-cost granular AC and AC fibers to be reused with less effort and energy. The chemical regeneration of activated carbon after Ni(II) ions adsorption was investigated in this study. Various desorbing solutions were used to recover adsorbed Ni(II) ions to AC. The concentration of the most effective desorbing solution was optimized. The adsorption efficiency of the regenerated AC using merely an acidic desorbing solution was reduced. Therefore, additional treatment after desorption using an acidic desorbing solution was carried out using sodium hydroxide solution. The optimum concentration of sodium hydroxide was determined. Using 3% HCl and H2SO4 as desorbing solutions, more than 80% of Ni(II) ions could be desorbed from AC. However, the readsorption efficiency of Ni(II) ions by AC was reduced to less than 50% after the first regeneration. By treating the regenerated AC from the acidic desorbing solution with 1 % NaOH, the efficiencies in the Ni(II) ions adsorption and desorption were fully recovered to almost 100%.
References
Genchi, G., Carocci, A., Lauria, G., Sinicropi, M.S., and Catalano, A.2020. Ni(II): Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health. 17(3). 1-21. doi: 10.3390/ijerph17030679. https://doi.org/10.3390/ijerph17030679
Xavier, A.L.P., Adarme, O.F.H., Furtado, L.M., Ferreira, G.M.D., da Silva, L.H.M., Gil, L.F., and Gurgel, L.V.A. 2018. Modeling adsorption of copper(II), cobalt(II) and Ni(II) metal ions from aqueous solution onto a new carboxylated sugarcane bagasse. J. Colloid Interface Sci. 516. 431-445. doi: 10.1016/j.jcis.2018.01.068. https://doi.org/10.1016/j.jcis.2018.01.068
Coman, V., Robotin, B., and Llea, P. 2013. Ni(II) recovery/removal from industrial wastes: a review. Resour. Conserv. Recycl. 73. 229-238. doi:10.1016/j.resconrec.2013.01.019. https://doi.org/10.1016/j.resconrec.2013.01.019
Das, Kusal K., Reddy, R. Chandramouli, Bagoji, Ishwar B., Das, Swastika, Bagali, Shrilaxmi, Mullur, Lata, Khodnapur, Jyoti P. and Biradar, M.S. 2019. Primary concept of Ni(II) toxicity - an overview. J. Basic Clin. Physiol. Pharmacol. 30(2). 141-152. doi: 10.1515/jbcpp-2017-0171. https://doi.org/10.1515/jbcpp-2017-0171
Khulbe, K.C., Matsuura, T. 2018. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 8(19). 1-30. doi: 10.1007/s13201-018-0661-6. https://doi.org/10.1007/s13201-018-0661-6
Zolfaghari, G. and Kargar, M. 2019. Nanofiltration and microfiltration for the removal of chromium, total dissolved solids, and sulfate from water. MethodsX. 6. 549-557. doi: 10.1016/j.mex.2019.03.012. https://doi.org/10.1016/j.mex.2019.03.012
Shrestha, S., Lee, S.H., and Lee, T.K. 2015. Micellar enhanced ultrafiltration (MEUF): activated carbon fiber (ACF) hybrid process using low surfactant concentration for zinc(II) removal from synthetic wastewater. Desalin. Water Treat. 54(4-5). 929-943. doi: 10.1080/19443994.2014.912160. https://doi.org/10.1080/19443994.2014.912160
Haghighi, M., Rahmani, F., Dehghani, R., Tehrani, A.M., Ashraf and Miranzadeh, M.B. 2017. Photocatalytic reduction of Cr (VI) in aqueous solution over ZnO/HZSM-5 nanocomposite: Optimization of ZnO loading and process conditions. Desalin. Water Treat. 58. 168-180. doi: 10.5004/dwt.2017.0145. https://doi.org/10.5004/dwt.2017.0145
Azizi, A. and Saien, J. 2018. Optimization of Cr(VI) Photocatalytic Reduction by UV/TiO2: Influence of Inorganic and Organic Species and Kinetic Study. Arch. Hyg. Sci. 7(2). 81-90. doi: 10.29252/ArchHygSci.7.2.81. https://doi.org/10.29252/ArchHygSci.7.2.81
Chaudhary, R and Singh, C. 2014. Removal of metal ions by means of solar oxidation processes based on pH, TiO2 and oxidants. Desalin. Water Treat. 52(7-9). 1263-1271. doi: 10.1080/19443994.2013.787552. https://doi.org/10.1080/19443994.2013.787552
Gabli, M., Smara, A., Mecibah, W., and Djellabi, R. 2020. Intensification of Ni(II) recovery from water using an electrically driven hybrid process: continuous electropermutation. Environ. Technol. 41(15). 2003-2012. doi: 10.1080/09593330.2018.1554005. https://doi.org/10.1080/09593330.2018.1554005
Khan, Z. U., Moronshing, M., Shestakova, M., Al-Othman, A., Sillanpää, M., Zhan, Z., Song, B., and Lei, Y. 2023. Electro-deionization (EDI) technology for enhanced water treatment and desalination: A review. Desalination. 548. 116254. doi: 10.1016/j.desal.2022.116254. https://doi.org/10.1016/j.desal.2022.116254
Hakim, A.N., Khoiruddin, K., Ariono, D., and Wenten, I.G. 2020. Ionic Separation in Electrodeionization System: Mass Transfer Mechanism and Factor Affecting Separation Performance. Sep. Purif. Rev. 49(4). 294-316. doi: 10.1080/15422119.2019.1608562. https://doi.org/10.1080/15422119.2019.1608562
Chen, X., Huang, G., and Wang, J. 2013. Electrochemical reduction/oxidation in the treatment of heavy metal wastewater. J. Mater. Eng (ME). 2(4). 161-164.
Borghe-ei S.M, Goodarzi J, MohseniM, Amouei A . 2015 Efficiency of Removing Chromium from Plating Industry Wastewater using the Electrocoagulation Method. Int. Arch. Health Sci. 2(2). 83-87. doi: 10.1061/(ASCE)HZ.2153-5515.0000170. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000170
Bazrafshan, E., Mohammadi, L., Ansari-Moghaddam, A., and Mahvi, A. H. 2015. Heavy metals removal from aqueous environments by electrocoagulation process- a systematic review. J. Environ. Health Sci. Eng. 13. 1-16. doi: 10.1186/s40201-015-0233-8. https://doi.org/10.1186/s40201-015-0233-8
Keerthi, Vinduja, V., and Balasubramanian, N. 2013. Removal of heavy metals by hybrid electrocoagulation and microfiltration processes. Environ. Technol. 34(17-20). 2897-2902. doi: 10.1080/09593330.2013.796005. https://doi.org/10.1080/09593330.2013.796005
de Oliveira da Mota, I., de Castro, J.A., de Góes Casqueira, R., and de Oliveira Junior, A.G. 2015. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 4(2). 109-113. doi: 10.1016/j.jmrt.2014.11.004. https://doi.org/10.1016/j.jmrt.2014.11.004
Li, X., Zhang, Q., and Yang, B. 2019. Co-precipitation with CaCO3 to remove heavy metals and significantly reduce the moisture content of filter residue. Chemosphere. 239. 124660. doi: 10.1016/j.chemosphere.2019.124660. https://doi.org/10.1016/j.chemosphere.2019.124660
Thaçi, B.S., Gashi, S.T. 2019. Reverse Osmosis Removal of Heavy Metals from Wastewater Effluents Using Biowaste Materials Pretreatment. Polish J. Environ. Sci. Stud. 28(1). 337-341. doi: 10.15244/pjoes/81268. https://doi.org/10.15244/pjoes/81268
Harharah, R., Abdalla, G., Elkhaleefa, A., Shigidi, I., and Harharah, H. 2022. A Study of Copper (II) Ions Removal by Reverse Osmosis under Various Operating Conditions. Separations. 9. 155. doi: 10.3390/separations9060155. https://doi.org/10.3390/separations9060155
Lumami, K.V., García, A.M., Sang, S.V., Buleng, N.T.E., Ndikumana, T., Musibono D.D., Van der Bruggen, B., and Luis, P. 2022. Evaluation of Commercial Reverse Osmosis and Nanofiltration Membranes for the Removal of Heavy Metals from Surface Water in the Democratic Republic of Congo. Clean Technol. 4(4). 1300-1316. doi: 10.3390/cleantechnol4040080. https://doi.org/10.3390/cleantechnol4040080
Hamid, M.F., Yusof, N., Ismail, N.M., and Azali, M.A. 2020. Role of Membrane Surface Charge and Complexation-Ultrafiltration for Heavy Metals Removal: A Mini Review. J. Memb. Separ. Tech. 24(1). 39-49. doi: 10.11113/amst.v24n1.170. https://doi.org/10.11113/amst.v24n1.170
Kumar, A., Balouch, A., Pathan, A., Abdullah, Jagirani, M.S., Mahar, A.M., Zubair, M. and Laghari, B. 2019. Remediation of Ni(II) ion from wastewater by applying various techniques: a review. Acta Chem. Malays. 3(1). 1-15. doi: 10.2478/acmy-2019-0001. https://doi.org/10.2478/acmy-2019-0001
Kyzas, G.Z., and Matis., K.A. 2018. Flotation in Water and Wastewater Treatment. Processes. 6(8). 116. doi: 10.3390/pr6080116. https://doi.org/10.3390/pr6080116
Ker€anen, A., Leivisk€a, T., Salakka, A., and Tanskanen, J. 2015. Removal of Ni(II) and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalin. Water Treat. 53. 2645-2654. doi: 10.1080/19443994.2013.868832. https://doi.org/10.1080/19443994.2013.868832
Yang, B., Li, C., Wang, J., Wei, W., Hao, B., Yang, L., Tang, J., and Zhang, C. 2020. Removal of Ni(II) ions from automobile industry wastewater using ion exchange resin: Characterization and parameter optimization. IOP Conf. Ser.: Earth Environ. Sci. 467. 012182. doi: 10.1088/1755-1315/467/1/012182. https://doi.org/10.1088/1755-1315/467/1/012182
Budak, T.. 2013. Removal of Heavy Metals from Wastewater Using Synthetic Ion Exchange Resin. Asian J. Chem. 25. 4207-4210. doi: 10.14233/ajchem.2013.13902. https://doi.org/10.14233/ajchem.2013.13902
Ahmad, N.A., Hassan, M. A. A., Noor, Z.Z., Evuti, A.M., and Danlami, J.M. 2014. Optimization of Ni(II) Removal from Electroless Plating Industry Wastewater using Response Surface Methodology. Jurnal Teknologi, 67(4). 33-40. doi: 10.11113/JT.V67.2789. https://doi.org/10.11113/jt.v67.2789
Kumar, P. S., Ramakrishnan, K., and Gayathri., R. 2010. Removal of Ni(II) from Aqueous Solutions by Ceralite IR 120 Cationic Exchange Resins. J. Eng. Sci. Technol. 5(2). 232-243.
Zewail, T. and Yousef, N. 2015. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex. Eng. J. 54(1). 83-90. doi: 10.1016/j.aej.2014.11.008. https://doi.org/10.1016/j.aej.2014.11.008
Qasem, N.A.A., Mohammed, R.H. and Lawal, D.U. 2021. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean Water. 4(1). 36. doi: 10.1038/s41545-021-00127-0. https://doi.org/10.1038/s41545-021-00127-0
Saleem, J., Shahid, U., Hijab, M., Mackey, H., Mckay, G. 2019. Production and applications of activated carbons as adsorbents from olive stones. Biomass Conv. Bioref. 9. 775-802. doi: 10.1007/s13399-019-00473-7. https://doi.org/10.1007/s13399-019-00473-7
Hagemann, Nikolas, Kurt Spokas, Hans-Peter Schmidt, Ralf Kägi, Marc Anton Böhler, and Thomas D. Bucheli. 2018. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon's ABCs. Water. 10(2). 182. doi: 10.3390/w10020182. https://doi.org/10.3390/w10020182
Andas, J., Rahman, M. L.A., and Yahya, M.S.M. 2017. Preparation and Characterization of Activated Carbon from Palm Kernel Shell. IOP Conference Series: Mater. Sci. Eng. 226. 012156. doi: 10.1088/1757-899X/226/1/012156. https://doi.org/10.1088/1757-899X/226/1/012156
Sousa Ribeiro, L.A. d., Rodrigues, L.A., and Thim, G.P. 2017. Preparation of Activated Carbon from Orange Peel and its Application for Phenol Removal. Int. J. Eng. Res. Sci. 3(3). 122-129.
Nahm, S.W., Shim, W.G., Park, Y., and Kim, S.C. 2012. Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J. 210. 500-509. doi: 10.1016/j.cej.2012.09.023. https://doi.org/10.1016/j.cej.2012.09.023
Li, Q., Qi, Y., and Gao, C. 2015. Chemical regeneration of spent powdered activated carbon used in decolorization of sodium salicylate for pharmaceutical industry. J. Clean. Prod. 86. 424-431. doi: 10.1016/j.jclepro.2014.08.008. https://doi.org/10.1016/j.jclepro.2014.08.008
Ding, Z., Hu, X., Wan, Y., Wang, S. and Gao, B. 2016. Removal of lead, copper, cadmium, zinc, and Ni(II) from aqueous solutions by alkali-modified biochar: batch and column tests. J. Ind. Eng. Chem. 33. 239-245. doi: 10.1016/j.jiec.2015.10.007. https://doi.org/10.1016/j.jiec.2015.10.007
Bogusz, A., Nowak, K., Stefaniuk, M., Dobrowolski, R. and Oleszczuk, P. 2017. Synthesis of biochar from residues after biogas production with respect to cadmium and Ni(II) removal from wastewater. J. Environ. Manage. 201. 268-276. doi: 10.1016/j.jenvman.2017.06.019. https://doi.org/10.1016/j.jenvman.2017.06.019
Choudhary, M., Kumar, R. and Neogi, S. 2020. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 392. 122441. doi: 10.1016/j.jhazmat.2020.122441. https://doi.org/10.1016/j.jhazmat.2020.122441
Iamsaard, K., Weng, C.H, Yen, L.T., Tzeng, J.H., Poonpakdee, C., Lin, Y.T. 2022. Adsorption of metal on pineapple leaf biochar: Key affecting factors, mechanism identification, and regeneration evaluation. Bioresour. Technol. 344. 126131. doi: 10.1016/j.biortech.2021.126131. https://doi.org/10.1016/j.biortech.2021.126131
Abdel-Ghani, N.T., El-Chaghaby, G.A. and Zahran, E.M. 2015. Cost Effective Adsorption of Aluminium and Iron from Synthetic and Real Wastewater by Rice Hull Activated Carbon (RHAC). Am. J. Analyt. Chem. 6(1). 71. doi: 10.4236/ajac.2015.61007. https://doi.org/10.4236/ajac.2015.61007
Kolodynska D., Krukowska J., and Thomas P. 2017. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon, Chem. Eng. J. 307. 353-363. doi: 10.1016/j.cej.2016.08.088. https://doi.org/10.1016/j.cej.2016.08.088
Fonseca-Correa, R.A., Giraldo, L., and Moreno-Piraján, J.C. 2019. Thermodynamic study of adsorption of Ni(II) ions onto carbon aerogels. Heliyon. 5(6). e01789. doi: 10.1016/j.heliyon.2019.e01789. https://doi.org/10.1016/j.heliyon.2019.e01789
Vakili, M., Rafatullah, M., Yuan, J., Zwain, H. M., Mojiri, A., Gholami, Z., Gholami, Fatemeh, W., Wang, W., Giwa, A. S., Yu, Y., Cagnetta, G., and Yu, G. 2021. Ni(II) ion removal from aqueous solutions through the adsorption process: a review. Rev. Chem. Eng. 37(6). 755-778. doi: 10.1515/revce-2019-0047. https://doi.org/10.1515/revce-2019-0047
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.