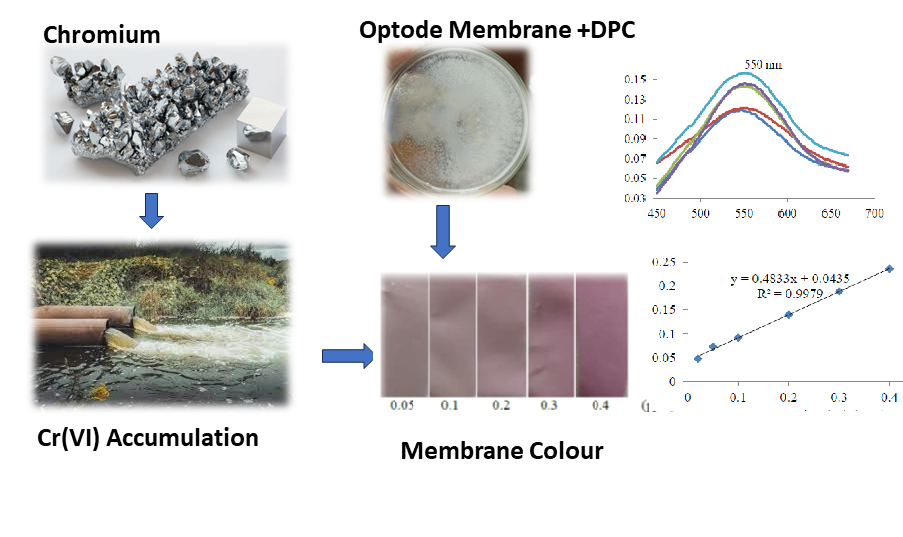
Detection of Hexavalent Chromium Ion in Water by Optode Membrane
DOI:
https://doi.org/10.55749/ijcs.v2i2.39Keywords:
Cellulose triacetate, Hexavalent chromium, Membrane, Optode, PollutantsAbstract
Chromium is a heavy metal that is often found as a water pollutant. High concentrations of hexavalent chromium (Cr(VI)) ion in nature are toxic and carcinogenic, so their presence in water needs to be monitored. Detection of heavy metals in water was carried out using an optical sensor membrane (optode). The optode was fabricated from cellulose triacetate polymer with plasticizers (oleic acid and acetophenone), aliquot 336, and the chromoionophore 1.5-diphenylcarbazide (DPC). The success synthesis of optode was evidenced by FTIR and SEM characterization. The optode performance produces a linear response in detecting Cr(VI) ion in the concentration range of 0.02-0.40 mg/L with an R2 of 0.9930, as well as the best conditioning at pH 3. The detection and quantitation limits are 0.0055 mg/L and 0.0165 mg/L. The sensitivity of the chromium optode was excellence, with a molar absorptivity value of 8.8303 × 106 M-1cm-1. The performance test results of the chromium optode were acceptable because they meet the specified requirements for minimum detection concentration value.
References
Łukasik, N., Wagner-Wysiecka, E., & Małachowska, A. 2019. Iron(iii)-selective materials based on a catechol-bearing amide for optical sensing. The Analyst. 144(9), 3119–3127. doi: https://doi.org/10.1039/C9AN00188C.
Ghaedi, M., Shahamiri, A., Hajati, S., & Mirtamizdoust, B. 2014. A novel PVC-membrane optical sensor for high sensitive and selective determination of Cu2+ ion based on synthesized (E)-N′-(pyridin-2-ylmethylene)isonicotin-ohydrazide. J. Mol. Liquids. 199, 483–488. doi: https://doi.org/10.1016/j.molliq.2014.07.013.
Isha, A., Yusof, N.A., Ahmad, M., Suhendra, D., Yunus, W.M.Z.W., & Zainal, Z. 2007. Optical fibre chemical sensor for trace vanadium (V) determination based on newly synthesized palm based fatty hydroxamic acid immobilized in polyvinyl chloride membrane. Spectro. Acta - Part A: Mol. and Biomol. Spectro. 67(5), 1398–1402. doi: https://doi.org/10.1016/j.saa.2006.10.031.
Scindia, Y.M., Pandey, A.K., Reddy, A.V.R., & Manohar, S.B. 2004. Chemically selective membrane optode for Cr(VI) determination in aqueous samples. J . Anal. Chem. Acta. 515(2), 311–321. doi: https://doi.org/10.1016/j.aca.2004.03.074.
Łukasik, N., & Wagner-Wysiecka, E. 2017. Salicylaldimine-based receptor as a material for iron(III) selective optical sensing. J. of Photochem. and Photobio. A: Chem. 346, 318–326. doi: https://doi.org/10.1016/j.jphotochem.2017.06.011.
Urek, S.K., Francic, N., Turel, M., & Lobnik, A. 2013. Sensing heavy metals using mesoporous-based optical chemical sensors. J. Nanomater. 2013, 1–13. doi: https://doi.org/10.1155/2013/501320.
El-Feky, H.H., El-Bahy, S.M., Hassan, A.M.E., & Amin, A.S. 2021. Utility of a novel optical sensor design for ultra-trace detection of chromium colorimetrically in real environmental samples. Int. J. Environ. Anal. Chem. 16, 4031-4048. doi: https://doi.org/10.1080/03067319.2021.1921759 .
Kong, F. & Ni, Y. 2009. Determination of Cr(VI) concentration in diluted samples based on the paper test strip method. Water Scie. and Technol. 60(12), 1088-1097. doi: https://doi.org/10.2166/wst.2009.741.
Oliveira, H. 2012. Chromium as an environmental pollutant: insights on induced plant toxicity. J. Botany. 375843, 1-8. doi: https://doi.org/10.1155/2012/375843.
Wiryawan, A., Suntari, R., Kusuma, Z., Retnowati, R., Burhan, R.Y.P., & Syekhfani. 2018. Method of analysis for determination of the chromium (Cr) species in water samples by spectrophotometry with diphenylcarbazide. J. Environ. Engin. Sustain. Technol. 5(1), 37-46. doi: https://doi.org/10.21776/ub.jeest.2018.005.01.6.
Government Regulation No. 22. 2001. Penyelenggaraan Perlindungan dan Pengelolaan Lingkungan Hidup. Jakarta: Ministry of State Secretariat
Suah, F.B.M., Ahmad, M., & Heng, L.Y. 2015. A novel polymer inclusion membranes based optode for sensitive determination of Al3+ ions. Spectro. Acta Part A: Mol. and Biomol. Spectro. 144, 81–87. doi: https://doi.org/10.1016/j.saa.2015.02.068
Douglas-Gould, W., King, M., Mohapatra, B.R., Cameron, R.A., Kapoor, A., & Koren, D.W. 2012. A critical review on destruction of thiocyanate in mining effluents. Minerals Eng. 34, 38–47. doi: https://doi.org/10.1016/j.mineng.2012.04.009
Verma C, Tapadia K, & Soni AB. 2017. Determination of iron (III) in food, biological and environmental samples. Food Chem. 221, 1415–1420. doi: https://doi.org/10.1016/j.foodchem.2016.11.011
Duffy G, Maguire I, Heery B, Gers P, Ducree J, & Regan F. 2018. ChromiSense: A colourimetric lab-on-a-disc sensor for chromium speciation in water. Talanta. 178, 392-399. doi: https://doi.org/10.1016/j.talanta.2017.09.066.
Vieira, M.G.A., Silva, M.A., Santos, L.O., & Beppu, M.M. 2011. Natural-based plasticizers and biopolymer films: a review. Euro. Polym. J. 47(3), 254–263. doi: https://doi.org/10.1016/j.eurpolymj.2010.12.011
Alfanaar, R., & Notario, D. 2019. Sintesis senyawa koordinasi astaxanthin dengan bantuan gelombang ultrasonik. J. Kimia dan Kemasan. 41(2), 88–94. doi: https://doi.org/10.24817/jkk.v41i2.3366.
Pavia, D.L., Lampman, G.M., Kriz, G.S., & Vyvyan, J.A. 2009. Introduction to Spectroscopy 4th Edition. Washington: Brooks/Cole Cengange.
Li, F., Sun, M., Cheng, Q., & Yang, B. 2016. Preparation and characterization of graphene oxide/cellulose triacetate forward osmosis membranes. MATEC Web. Conf. 67, 1–6. doi: https://doi.org/10.1051/matecconf/20166701015.
Ingole, P.G., Kim, K.H., Park, C.H., & Choi, W.K., 2014. Preparation, modification and characterization of polymeric hollow fiber membranes for pressure retarded osmosis. RSC Adv. 4(93). 1-5. doi: https://doi.org/10.1039/C4RA07619B.
Xing, X.Y., Gu, L., Jin, Y., Sun, R., Xie, M., & Wu, Q. 2019. Fabrication and characterization of cellulose triacetate porous membranes by combined nonsolvent-thermally induced phase separation. Cellulose. 26(1002), 1-16. doi: https://doi.org/10.1007/s10570-019-02347-7
Rastegarzadeh, S., Nahid, P., & Zahra, J. 2013. Design of a sensitive membrane optode for iron determination. Inst. Sci. and Tech. 41(3), 290–300. doi: https://doi.org/10.1080/10739149.2012.729242
Lace, A., Ryan, D., Bowkett, M., & Cleary, J. 2019. Chromium monitoring in water by colorimetry using optimised 1,5-diphenylcarbazide method. Int. J. Environ. Res. Public Health. 16(10), 1803-18017. doi: https://doi.org/10.3390/ijerph16101803.
Chong, K.W. & Cho, K.H. 2023. Chemical Regeneration of Activated Carbon After Adsorption of Ni(II) Ions. Indones. J. Chem. Stud. 2(1), 1–8. doi: https://doi.org/10.55749/ijcs.v2i1.20.
Association of Official Analytical Chemist. 2013. Official Method of Analysis of AOAC International 20th Ed. Washington: AOAC Pr.
Chikanbanjar, N., Semwal, N., & Jyakhwa, U. 2020. A review article on analytical method validation, J. Pharma. Innov. 1(1), 48-58.
Regulation of the Minister of Health of the Republic of Indonesia Number 32. 2017. Standar Baku Mutu Kesehatan Lingkungan dan Persyaratan Kesehatan Air untuk Keperluan Higiene Sanitasi, Kolam renang, Solus Per Aqua, dan Pemandian Umum. Jakarta: Ministry of Health.
Firooz, A.R., Movahedi, M., & Sabzyan, H. 2019. A new selective optode for the determination of iron(III) based on the immobilization of morin on triacetylcellulose: A combined experimental and computational study. J. Mat. Scie. & Eng. 94, 410-416. doi: https://doi.org/10.1016/j.msec.2018.09.031.
Harmita. 2004. Petunjuk pelaksanaan validasi metode dan cara perhitungannya. Majalah Ilmu Kefarmasian. 1(3), 117-135. doi: https://doi.org/10.7454/psr.v1i3.3375.
Harvey, D. 2016. Analytical Chemistry 21. New York: McGraw-Hill Companies.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.