Temperature Combustion Profiles of KNO₃, CsNO₃, and NaNO₃-Based Energetic Materials at Atmospheric Pressure
DOI:
https://doi.org/10.55749/ijcs.v4i2.74Keywords:
Combustion, CsNO₃-based sample, KNO₃-based sample, NaNO₃-based sample, Temperature profileAbstract
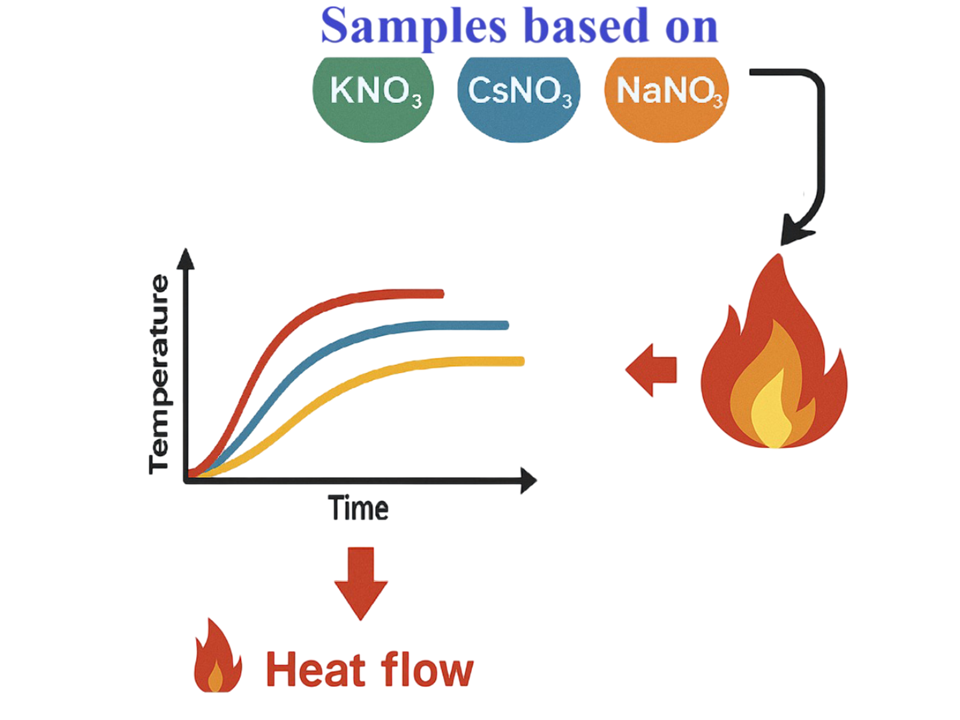
This study investigated the thermal properties of energetic materials composed of alkaline metal nitrates, specifically KNO3, CsNO3, and NaNO3, at atmospheric pressure. This study primarily evaluated the burning surface temperature and characterized the heat release behavior of the studied compositions during combustion. To determine the thermal output, temperature profiles were obtained using thermocouple wires on the combustion surface. Given the heterogeneous structure of these formulations and the reactivity of alkali metals during combustion, finding a consistent temperature signatures presented an analytical challenge. All the tested samples exhibited very high surface combustion temperatures. The KNO3-based sample exhibited a higher maximum heat-flow density than the NaNO3-based formulation. Variations in the temperature profiles resulted from the differences in composition structure and combustion reactivity. This study showed the effect of nitrate type on the thermal performance of energetic materials. These results clarified the influence of nitrate identity on heat transfer and combustion efficiency in nitrate-based pyrotechnic formulations, and may inform the design of thermally stable, high-performance propellants and thermal generator formulations.
References
[1] Freeman E.S. 1957. The kinetics of the thermal decomposition of potassium nitrate and of the reaction between potassium nitrite and oxygen1a. J. Am. Chem. Soc. 79. 838-842.
[2] Turcotte R., Fouchard R., Turcotte A.-M., Jones D. 2003. Thermal analysis of black powder. J. Therm. Anal. Calorim. 73. 105-118. doi: https://doi.org/10.1023/A:1025181424038
[3] Pouretedal H.R., Ebadpour R. 2014. Application of non-isothermal thermogravimetric method to interpret the decomposition kinetics of NaNO₃, KNO₃, and KClO₄. Int. J. Thermophys. 35(5). 942-951. doi: https://doi.org/10.1007/s10765-014-1636-y
[4] Hosseini, S. and Eslami, A. 2010. Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems. J. Therm. Anal. Calorim. 101(3). 1111-1119. doi: https://doi.org/10.1007/s10973-010-0813-x
[5] Gordon, S. and Campbell, C. 1955. Differential thermal analysis of inorganic compounds. Anal. Chem. 27(7). 1102-1109.
[6] Stern, K.H. 1972. High temperature properties and decomposition of inorganic salts part 3, nitrates and nitrites. J. Phys. Chem. Ref. Data. 1(3). 747-772. doi: https://doi.org/10.1063/1.3253104
[7] Mohammad, M.B., Brooks, G.A. and Rhamdhani, M.A. 2018. Premelting, melting, and degradation properties of molten alkali nitrates: LiNO₃, NaNO₃, KNO₃, and binary NaNO₃-KNO₃. Metall. Mater. Trans. B. 49(3). 1482-1498. doi: https://doi.org/10.1007/s11663-018-1205-z
[8] Bond, B.D. and Jacobs, P.W.M. 1966. The thermal decomposition of sodium nitrate. J. Chem. Soc. A. 1265-1268. doi: https://doi.org/10.1039/J19660001265
[9] Kouassi, E.K., Manouan, M.W., Zoro, E., Boa, D., Benigni, P., Zamali, H., Rogez, J. and Hellali, D. 2021. Experimental investigation and thermodynamic evaluation of the CsNO₃-LiNO₃-NaNO₃ ternary system. J. Alloys Compd. 864. 158131. doi: https://doi.org/10.1016/j.jallcom.2020.158131
[10] Freeman, E.S. 1956. The kinetics of the thermal decomposition of sodium nitrate and of the reaction between sodium nitrite and oxygen. J. Phys. Chem. 60(11). 1487-1493.
[11] Singh, H. and Bhaskara Rao, R., 1990. Temperature sensitivity of magnesiu‐sodium nitrate propellants. Prop., Explos., Pyrotech. 15(6). 250-253. doi: https://doi.org/10.1002/prep.19900150605
[12] Singh, H. and Bhaskara Rao, R. 1992. Effect of particle size on combustion of magnesium-sodium nitrate propellants. Combust. Sci. Technol. 81(4-6). 233-242. doi: https://doi.org/10.1080/00102209208951804
[13] Silin, N.A., Kashporov, L.Y., Sheludyak, Y.E., Asmatullov, Z.E., Raspopin, A.G. and Grineeva, R.A. 1992. Combustion rate functional dependence on various variables for the Mg+ NaNO₃ mixture. Combust. Explos. Shock Waves. 28(5). 474-481. doi: https://doi.org/10.1007/BF00755717
[14] Singh, H., Somayajulu, M.R. and Rao, R.B. 1989. A study on combustion behavior of magnesium-sodium nitrate binary mixtures. Combust. Flame. 76(1). 57-61. doi: https://doi.org/10.1016/0010-2180(89)90077-1
[15] Guo, Z., Guan, H., Shi, C. and Zhou, B. 2024. Study of combustion characteristics of Magnesium/Strontium Nitrate and Magnesium/Sodium Nitrate pyrotechnics under low pressure environment. Combust. Sci. Technol. 196(15). 3365-3381. doi: https://doi.org/10.1080/00102202.2023.2175608
[16] Pouretedal, H.R. and Ravanbod, M. 2015. Kinetic study of ignition of Mg/NaNO₃ pyrotechnic using non-isothermal TG/DSC technique. J. Therm. Anal. Calorim. 119(3). 2281-2288. doi: https://doi.org/10.1007/s10973-014-4330-1
[17] Denisyuk, A.P., Duy Tuan, N. and Sizov, V.A. 2022. Combustion behavior of the inorganic nitrates‐based compositions. part ii: effect of Al and Al‐Mg alloy on burning rate. Prop., Explos., Pyrotech. 47(5). e202100145. doi: https://doi.org/10.1002/prep.202100145
[18] Denisyuk, A.P., Duy Tuan, N. and Sizov, V.A. 2020. Combustion behavior of the inorganic nitrates‐based compositions part I. Prop., Explos., Pyrotech. 45(9). 1382-1387. doi: https://doi.org/10.1002/prep.202000053
[19] Tuan, N.D., Denisuyk, A.P., Khai, D.M. and Sizov, V.A., 2023. Influence of some additives on burning rate of KNO₃-based compositions. Vietnam J. Sci. Technol. 61(6). 1000-1009. doi: https://doi.org/10.15625/2525-2518/17077
[20] Denisyuk, A., Rusin, D. and Long, N. 2007. Mechanism of combustion of fire-extinguishing propellants based on potassium nitrate. Dokl. Phys. Chem. 414(1). 99. doi: https://doi.org/10.1134/S0012501607050016
[21] Jiang, H., Wang, J., Wu, S., Yuan, Z., Hu, Z., Wu, R. and Liu, Q. 2012. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stab. 97(8). 1527-1533. doi: https://doi.org/10.1016/j.polymdegradstab.2012.04.016
[22] Shtessel', E.A. and Dorozhevets, I.N. 1990. Combustion of heterogeneous condensed systems in the presence of chemical transport reactions. Combust. Explos. Shock Waves. 26(1). 52-59. doi: https://doi.org/10.1007/BF00742272
[23] Bakhman, N.N. and Belyaev, A.F. 1967. Combustion of heterogeneous condensed systems (No. RPETRANS19). https://apps.dtic.mil/sti/html/tr/AD0670440/
[24] Dukhanin, G.P. and Lopatin, S.I. 2011. Mass-spectrometric examination of vaporization of sodium nitrite and sodium and potassium nitrates. Russ. J. Appl. Chem. 84(2). 184-189. doi: https://doi.org/10.1134/S1070427211020030
[25] Ichikawa, K. and Matsumoto, T. 1983. The heat capacities of lithium, sodium, potassium, rubidium, and caesium nitrates in the solid and liquid states. Bull. Chem. Soc. Jpn. 56(7). 2093-2100.
[26] Maeso, M.J. and Largo, J. 1993. The heat capacities of LiNO₃ and CsNO₃ from 350 to 700 K. Thermochim. Acta. 222(2). 195-201. doi: https://doi.org/10.1016/0040-6031(93)80552-L
[27] Jriri, T., Rogez, J., Bergman, C. and Mathieu, J.C. 1995. Thermodynamic study of the condensed phases of NaNO₃, KNO₃ and CsNO3 and their transitions. Thermochim. Acta. 266. 147-161. doi: https://doi.org/10.1016/0040-6031(95)02337-2
[28] Korabel’nikov, D.V. and Zhuravlev, Y.N. 2013. Theoretical study of the thermodynamic properties of lithium, sodium, and potassium nitrates. Phys. Solid State. 55(8). 1765-1772.2. doi: https://doi.org/10.1134/S1063783413080155
[29] Takahashi, Y., Sakamoto, R. and Kamimoto, M., 1988. Heat capacities and latent heats of LiNO₃, NaNO₃, and KNO₃. Int. J. Thermophys. 9(6). 1081-1090. doi: https://doi.org/10.1007/BF01133275
[30] Deshpande, V.V., Karkhanavala, M.D. and Rao, U.R.K., 1974. Phase transitions in potassium nitrate. J. Therm. Anal. Calorim. 6(6). 613-621. doi: https://doi.org/10.1007/bf01911781
[31] Wypych, G., 2004. Handbook of plasticizers. ChemTec Publishing.
[32] Godwin, A.D., 2024. Plasticizers. In Applied plastics engineering handbook (pp. 595-618). William Andrew Publishing. Doi: https://doi.org/10.1016/B978-0-323-88667-3.00031-X
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.