Magnetically Separable Humic Acid-Chitin Based Adsorbent as Pb(II) Uptake in Synthetic Wastewater
DOI:
https://doi.org/10.55749/ijcs.v2i1.22Keywords:
Adsorption, Fe3O4/HA-Chitin, Humic acid, Pb(II) uptakeAbstract
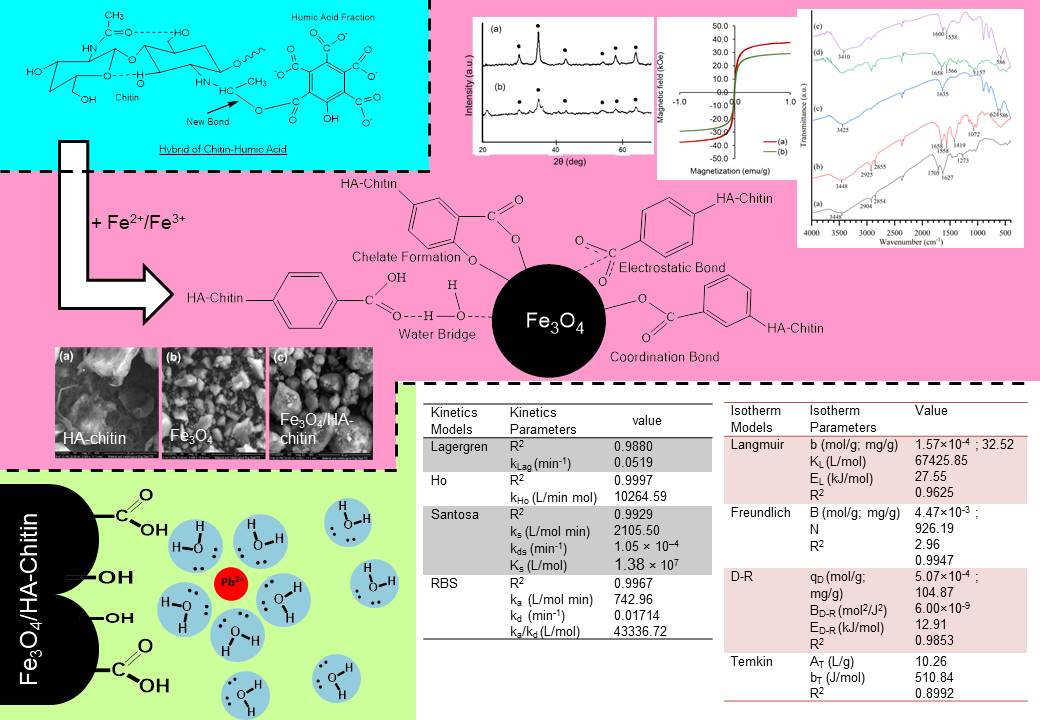
Modification of humic acid (HA) from the peat soil of Jambi province, Indonesia with chitin and magnetite to form Fe3O4/HA-chitin has been successfully carried out. The successful synthesis was identified from characterization with functional group analysis, crystal analysis, magnetic strength measurement, and morphological and elemental analysis. The application of Fe3O4/HA-chitin to adsorb Pb(II) ion was analyzed using the Lagergren, Ho, Santosa, and RBS kinetics models (kinetics study) and the Langmuir, Freundlich, Dubinin-Radushkevich (DR), and Temkin isotherm model (isotherm study). The kinetics study followed the Ho model (pseudo-second order) with R2 and kHo of 0.9997 and 10264.59 g/mol min, respectively. The results of the data applicable to the Freundlich model showed that several sites were capable of multilayer adsorption (B) with a large enough adsorption capacity of 929.19 mg/g (about 28 times higher than the monolayer adsorption of Langmuir data). However, the outermost layer had a feeble adsorption energy of 0.51 kJ/mol, as measured by Temkin's adsorption energy. In the layer between the first layer (Langmuir) and the outermost layer (Freundlich), the DR isotherm was measured at a capacity of 104.87 mg/g (qD, the 3rd layer of the first layer) the adsorption energy was measured at 12.91 kJ/mol. A cross-study on the prediction of adsorption energy using the Santosa and RBS kinetics models showed that the RBS model had an adsorption energy value (26.45 kJ/mol) that was closer to the adsorption energy value of the Langmuir isotherm (27.55 kJ/mol).
References
Ziegfeld, R.L. 1964. Importance and uses of lead. Arch. Environ. Health. 8(2). 202-212. doi: 10.1080/00039896.1964.10663657. https://doi.org/10.1080/00039896.1964.10663657
Qin, D., Chen, A., Mamat, X., Li, Y., Hu, X., Wang, P., Cheng, H., Dong, Y., & Hu, G. 2019. Double-shelled yolk-shell Si@C microspheres based electrochemical sensor for determination of cadmium and lead ions. Anal. Chim. Acta. 1078. 32-41. doi: 10.1016/j.aca.2019.06.011. https://doi.org/10.1016/j.aca.2019.06.011
Wang, Y. & Liu, R. 2018. H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions. Sci. Total Environ. 628-629. 1139-1148. doi: 10.1016/j.scitotenv.2018.02.137. https://doi.org/10.1016/j.scitotenv.2018.02.137
Awual, M.R. & Hasan, M.M. 2014. Novel conjugate adsorbent for visual detection and removal of toxic lead(II) ions from water. Microporous Mesoporous Mater. 196. 261-269. doi: 10.1016/j.micromeso.2014.05.021. https://doi.org/10.1016/j.micromeso.2014.05.021
Rasaki, S.A., Thomas, T., & Yang, M. 2019. Co-precipitation strategy for engineering pH-tolerant and durable ZnO@MgO nanospheres for efficient, room-temperature, chemisorptive removal of Pb(II) from water. J. Environ. Chem. Eng. 7(2). 1-12. doi: 10.1016/j.jece.2019.103019. https://doi.org/10.1016/j.jece.2019.103019
Cao, Y., Xiao, W., Shen, G., Ji, G., Zhang, Y., Gao, C., & Han, L. 2019. Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 273. 70-76. doi: 10.1016/j.biortech.2018.10.065. https://doi.org/10.1016/j.biortech.2018.10.065
Hou, Q., Zhou, H., Zhang, W., Chang, Q., Yang, J., Xue, C., & Hu, S. 2021. Boosting adsorption of heavy metal ions in wastewater through solar-driven interfacial evaporation of chemically-treated carbonized wood. Sci. Total Environ. 759. 144317. doi: 10.1016/j.scitotenv.2020.144317. https://doi.org/10.1016/j.scitotenv.2020.144317
Roy Choudhury, P., Majumdar, S., Sahoo, G.C., Saha, S., & Mondal, P. 2018. High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem. Eng. J. 336. 570-578. doi: 10.1016/j.cej.2017.12.062. https://doi.org/10.1016/j.cej.2017.12.062
Hoang, M.T., Pham, T.D., Verheyen, D., Nguyen, M.K., Pham, T.T., Zhu, J., & Van der Bruggen, B. 2020. Fabrication of thin film nanocomposite nanofiltration membrane incorporated with cellulose nanocrystals for removal of Cu(II) and Pb(II). Chem. Eng. Sci. 228. 115998. doi: 10.1016/j.ces.2020.115998. https://doi.org/10.1016/j.ces.2020.115998
Zhang, J., Li, Y., Xie, X., Zhu, W., & Meng, X. 2019. Fate of adsorbed Pb(II) on graphene oxide under variable redox potential controlled by electrochemical method. J. Hazard. Mater. 367. 152-159. doi: 10.1016/j.jhazmat.2018.12.073. https://doi.org/10.1016/j.jhazmat.2018.12.073
Ngana, B.N., Seumo, P.M.T., Sambang, L.M., Dedzo, G.K., Nanseu-Njiki, C.P., & Ngameni, E. 2021. Grafting of reactive dyes onto lignocellulosic material: Application for Pb(II) adsorption and electrochemical detection in aqueous solution. J. Environ. Chem. Eng. 9(1). 104984. doi: 10.1016/j.jece.2020.104984. https://doi.org/10.1016/j.jece.2020.104984
Wang, Z., Xu, J., Yellezuome, D., & Liu, R. 2021. Effects of cotton straw-derived biochar under different pyrolysis conditions on Pb (II) adsorption properties in aqueous solutions. J. Anal. Appl. Pyrolysis. 157. 105214. doi: 10.1016/j.jaap.2021.105214. https://doi.org/10.1016/j.jaap.2021.105214
Ma, Y., Deng, Z., Li, Z., Lin, Q., Wu, Y., & Dou, W. 2021. Adsorption characteristics and mechanism for K2Ti4O9 whiskers removal of Pb(II), Cd(II), and Cu(II) cations in wastewater. J. Environ. Chem. Eng. 9(5). 106236. doi: 10.1016/j.jece.2021.106236. https://doi.org/10.1016/j.jece.2021.106236
Qi, K., Lu, N., Zhang, S., Wang, W., Wang, Z., & Guan, J. 2021. Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: Adsorption and combined toxicity in comparison to natural solid substrates. J. Hazard. Mater. 411. 125115. doi: 10.1016/j.jhazmat.2021.125115. https://doi.org/10.1016/j.jhazmat.2021.125115
Wang, W., Wu, G., Zhu, T., Yang, Y., & Zhang, Y. 2021. Synthesis of -thiazole Schiff base modified SBA-15 mesoporous silica for selective Pb(II) adsorption. J. Taiwan Inst. Chem. Eng. 125. 349-359. doi: 10.1016/j.jtice.2021.06.004. https://doi.org/10.1016/j.jtice.2021.06.004
Nata, I.F., Wicakso, D.R., Mirwan, A., Irawan, C., Ramadhani, D., & Ursulla. 2020. Selective adsorption of Pb(II) ion on amine-rich functionalized rice husk magnetic nanoparticle biocomposites in aqueous solution. J. Environ. Chem. Eng. 8(5). 104339. doi: 10.1016/j.jece.2020.104339. https://doi.org/10.1016/j.jece.2020.104339
Santosa, S.J., Siswanta, D., Kurniawan, A., & Rahmanto, W.H. 2007. Hybrid of chitin and humic acid as high performance sorbent for Ni(II). Surf. Sci. 601(22). 5155-5161. doi: 10.1016/j.susc.2007.04.163. https://doi.org/10.1016/j.susc.2007.04.163
Xu, H., Hu, X., Chen, Y., Li, Y., Zhang, R., Tang, C., & Hu, X. 2021. Cd(II) and Pb(II) absorbed on humic acid-iron-pillared bentonite: Kinetics, thermodynamics and mechanism of adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 612. 126005. doi: 10.1016/j.colsurfa.2020.126005. https://doi.org/10.1016/j.colsurfa.2020.126005
Ngatijo, N., Basuki, R., Rusdiarso, B., & Nuryono, N. 2020. Sorption-desorption profile of Au (III) onto silica modified quaternary amines (SMQA) in gold mining effluent. J. Environ. Chem. Eng. 8(3). 103747. doi: 10.1016/j.jece.2020.103747. https://doi.org/10.1016/j.jece.2020.103747
Basuki, R., Santosa, S.J., & Rusdiarso, B. 2017. The novel kinetics expression of Cadmium (II) removal using green adsorbent horse dung humic acid (Hd-Ha). AIP Conf. Proc. 1823(1). 020001. doi: 10.1063/1.4978074. https://doi.org/10.1063/1.4978074
Santosa, S.J., Siswanta, D., Sudiono, S., & Sehol, M. 2007. Synthesis and utilization of chitin-humic acid hybrid as sorbent for Cr(III). Surf. Sci. 601(22). 5148-5154. doi: 10.1016/j.susc.2007.04.161. https://doi.org/10.1016/j.susc.2007.04.161
Head, M.J. & Zhou, W.J. 2000. Evaluation of NaOH leaching techniques to extract humic acids from palaeosols. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms. 172(1-4). 434-439. doi: 10.1016/S0168-583X(00)00221-4. https://doi.org/10.1016/S0168-583X(00)00221-4
Fatnah, N., Azizah, D., & Cahyani, M.D. 2020. Synthesis of Chitosan from Crab's Shell Waste (Portunus pelagicus) in Mertasinga-Cirebon. 422. 370-375. doi: 10.2991/assehr.k.200323.152. https://doi.org/10.2991/assehr.k.200323.152
Zuorro, A., Cassiani-Cassiani, D., Meza-González, D.A., Moreno-Sader, K.A., & González-Delgado, Á.D. 2020. Evaluation of shrimp waste valorization combining computer-aided simulation and numerical descriptive inherent safety technique (NuDIST). Appl. Sci. 10(15). 5339. doi: 10.3390/APP10155339. https://doi.org/10.3390/app10155339
Kiprop, A.K., J-Coumon, M.-C., Pourtier, E., Kimutai, S., & Kirui, S. 2013. Synthesis of Humic and Fulvic Acids and their Characterization using Optical Spectroscopy ( ATR-FTIR and UV-Visible ). Int. J. Appl. Sci. Technol. 3(8). 28-35.
Santoso, U.T., Umaningrum, D., Irawati, U., & Nurmasari, R. 2010. Immobilization of Humic Acid on Chitosan using Protected Cross-Linking Reaction Method and Its Application as Sorbent for Pb(II), Cd(II), AND Cr(III). Indones. J. Chem. 8(2). 177-183. doi: 10.22146/ijc.21620. https://doi.org/10.22146/ijc.21620
Ren, G., Yang, L., Zhang, Z., Zhong, B., Yang, X., & Wang, X. 2017. A new green synthesis of porous magnetite nanoparticles from waste ferrous sulfate by solid-phase reduction reaction. J. Alloys Compd. 710. 875-879. doi: 10.1016/j.jallcom.2017.03.337. https://doi.org/10.1016/j.jallcom.2017.03.337
Liu, J.F., Zhao, Z.S., & Jiang, G. Bin. 2008. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 42(18). 6949-6954. doi: 10.1021/es800924c. https://doi.org/10.1021/es800924c
Kokubo, T. 1991. Recent progress in glass-based materials for biomedical applications. J. Ceram. Soc. Japan. Int. ed. 99(10). 937-944. https://doi.org/10.2109/jcersj.99.965
Lenders, J.J.M., Mirabello, G., & Sommerdijk, N.A.J.M. 2016. Bioinspired magnetite synthesis via solid precursor phases. Chem. Sci. 7(9). 5624-5634. https://doi.org/10.1039/C6SC00523C
Liu, S., Sun, J., Yu, L., Zhang, C., Bi, J., Zhu, F., Qu, M., Jiang, C., & Yang, Q. 2012. Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules. 17(4). 4604-4611. doi: 10.3390/molecules17044604. https://doi.org/10.3390/molecules17044604
Santosa, S.J. 2014. Sorption kinetics of Cd(II) species on humic acid-based sorbent. Clean - Soil, Air, Water. 42(6). 760-766. doi: 10.1002/clen.201200684. https://doi.org/10.1002/clen.201200684
Rusdiarso, B., Basuki, R., & Santosa, S.J. 2016. Evaluation of Lagergren kinetics equation by using novel kinetics expression of sorption of Zn 2+ onto horse dung humic acid (HD-HA). Indones. J. Chem. 16(3). 338-346. doi: 10.22146/ijc.1158. https://doi.org/10.22146/ijc.21151
Basuki, R., Ngatijo, Santosa, S.J., & Rusdiarso, B. 2018. Comparison the new kinetics equation of noncompetitive sorption Cd(II) and Zn(II) onto green sorbent horse dung humic acid (HD-HA). Bull. Chem. React. Eng. Catal. 13(3). 475-488. doi: 10.9767/bcrec.13.3.1774.475-488. https://doi.org/10.9767/bcrec.13.3.1774.475-488
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.