Temperature-influenced Bulk Emulsion (BE) Demulsification Method as a PIBSA-MEA Emulsifier Durability Test in Blasting Environments under 100 °C
DOI:
https://doi.org/10.55749/ijcs.v3i1.44Keywords:
Bulk Emulsion (BE), Demulsification, EmusifierAbstract
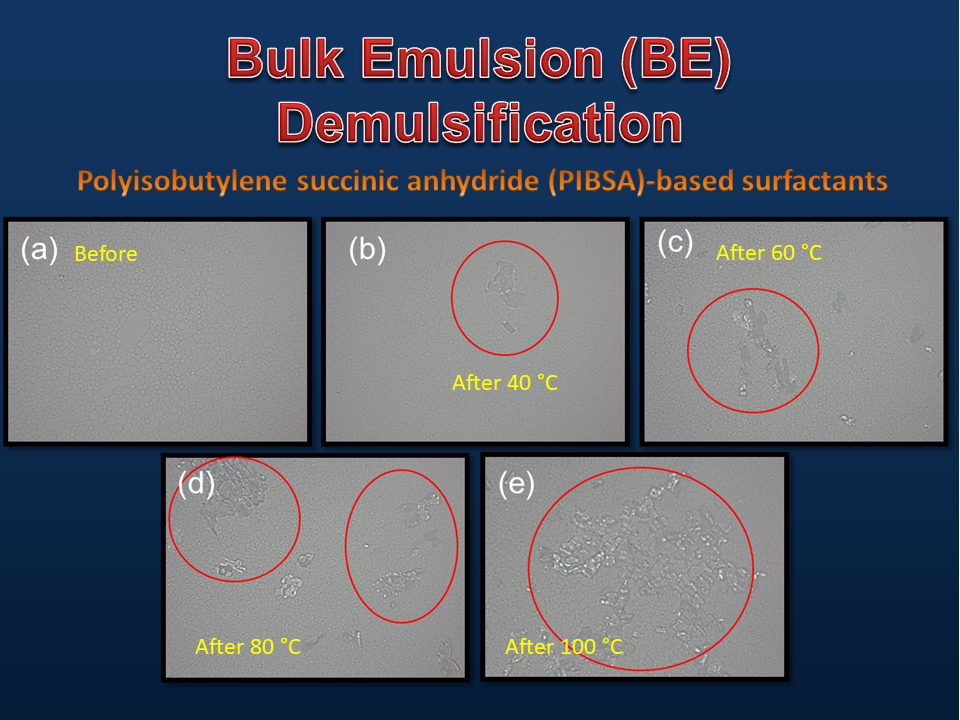
This research attempts to provide a better method, examine more effective temperatures for testing emulsifiers, and determine the demulsification limit that indicates emulsifier durability. This experiment was conducted by varying the temperature (40, 60, 80, and 100 °C) for heating the product with a test time of 1, 2, 4, and 6 h, then detected using formaldehyde titration to determine the highest level of demulsification of ammonium nitrate (AN) salt at each temperature in the product. The results showed that 100 °C was the most effective and representative temperature for testing the durability of the emulsifier with the highest level of demulsification from the other temperatures. This was indicated by the weight of AN salt that came out of the emulsion reaching 2.05 g from 20 g of emulsion or about 10.25% of the total weight of the product within 6 h. Emulsifiers with AN levels below 2.05 g (10.25%) were considered to pass the test and could be used for further production or analysis. This new test method was expected that bulk emulsion manufacturers would be faster in eliminating PIBSA-base (Polyisobutylene succinic anhydride-base) emulsifier products widely used by emulsifier manufacturers in manufacturing BE. This was due to it only focusing on the ability of emulsifiers to hold the product in high-temperature exposure so that it remained unified and not demulsified.
References
Van der Merwe, M.M., Landman, M., van Rooyen, P.H., Jordaan, J.H., Otto, D.P. 2017. Structural assignment of commercial polyisobutylene succinic anhydride-based surfactants. J. Surfactants Deterg. 20. 193-205. doi: https://doi.org/10.1007/s11743-016-1893-9.
Koch, E.C. 2012. Metal-fluorocarbon based energetic materials. John Wiley & Sons.
Pearton, S.P. 2015. The application of pumpable emulsions in narrow-reef stoping. J. South. Afr. Inst. Min. Metall. 115(6). 489-497.
Khomenko, O., Kononenko, M., Myronova, I., Savchenko, M. 2019. Application of the emulsion explosives in the tunnels construction. In E3S Web Conf. 123. 01039. EDP Sciences. doi: https://doi.org/10.1051/e3sconf/201912301039.
Mertuszka, P., & Kramarczyk, B. 2018. The impact of time on the detonation capacity of bulk emulsion explosives based on Emulinit 8L. Propellants Explos. Pyrotech. 43(8). 799-804. doi: https://doi.org/10.1002/prep.201800062.
Schramm, L.L. 2014. Emulsions, foams, suspensions, and aerosols: microscience and applications. John Wiley & Sons.
Tadros, T.F. 2014. Formulation of disperse systems: Science and Technology. John Wiley & Sons.
Sopianti, D.S., Ricki, A., Haque, A.F. 2021. Variasi ekstrak etanol biji kebiul (Caesalpinia Bonduc (L). Roxb) pada formulasi sediaan emulsi M/A. JIIS. 6(1). 11-20. doi: https://doi.org/10.36387/jiis.v6i1.568.
Kovalchuk, K., & Masalova, I. 2012. Factors influencing the crystallisation of highly concentrated water-in-oil emulsions: A DSC study. S. Afr. J. Sci. 108(3). 1-5. doi: https://doi.org/10.10520/EJC97225.
Pradhan, M. 2010. Sleep time: Its consequences on performance of bulk emulsion explosive. J. Sci. Ind. Res. 69. 125–128.
Zhang, H., Gao, D., Salehi, S., & Guo, B. 2014. Effect of fluid temperature on rock failure in borehole drilling. J. Eng. Mech. 140(1). 82-90. doi: 10.1061/(ASCE)EM.1943-7889.0000648.
Basuki, R., Yusnaidar, Y., & Rusdiarso, B. 2018. Different style of Langmuir isotherm model of non-competitive sorption Zn (II) and Cd (II) onto horse dung humic acid (HD-HA). AIP Conf. Proc. 2026(1). 020009. doi: https://doi.org/10.1063/1.5064969.
Santos, J.S., Reis, C., Reis, E.L., de Jesus, R.M., dos Reis, L. G.T., Matias, A.A. 2020. Determination of ammoniacal nitrogen in samples of food, soil, fertilizers and water based on the reaction with formaldehyde. J. Eng. Exact Sci. 6(5). 0647-0654. doi: https://doi.org/10.18540/jcecvl6iss5pp0647-0654.
Kebukawa, Y., & Cody, G.D. 2015. A kinetic study of the formation of organic solids from formaldehyde: Implications for the origin of extraterrestrial organic solids in primitive Solar System objects. Icarus. 248 412-423. doi: https://doi.org/10.1016/j.icarus.2014.11.005.
Xie, X.H., Feng, Y.Q., Liu, S.H., Zhu, J. 2020. Thermal behavior and stability of emulsion explosives in the presence of ferrous ion. J. Therm. Anal. Calorim. 139. 999-1006. doi: https://doi.org/10.1007/s10973-019-08494-0.
Cui, Z., Ge, F., Lin, Y., Wang, L., Lei, L., Tian, H., Yu, M., Wang, X. 2018. Corrosion behavior of AZ31 magnesium alloy in the chloride solution containing ammonium nitrate. Electrochim. Acta. 278. 421-437. doi: https://doi.org/10.1016/j.electacta.2018.05.059.
Saad, M. A., Kamil, M., Abdurahman, N. H., Yunus, R. M., & Awad, O. I. (2019). An overview of recent advances in state-of-the-art techniques in the demulsification of crude oil emulsions. Processes. 7(7). 470. doi: https://doi.org/10.3390/pr7070470.
Luo, K.M., Lin, S.H., Chang, J.G., Huang, T.H. 2002. Evaluations of kinetic parameters and critical runaway conditions in the reaction system of hexamine-nitric acid to produce RDX in a non-isothermal batch reactor. J. Loss Prev. Process Ind. 15(2). 119-127. doi: https://doi.org/10.1016/S0950-4230(01)00027-4.
Wang, F., Ma, H., & Shen, Z. (2017). Explosion performance of high-temperature degraded emulsion explosives. Propellants Explos. Pyrotech. 42(11). 1325-1332. doi: https://doi.org/10.1002/prep.201700052.
Zhao, H.R., Wu, J., Xu, M. X., & Zhang, K.M. 2021. Advances in the rheology of emulsion explosive. J. Mol. Liq. 336, 116854. doi: https://doi.org/10.1016/j.molliq.2021.116854.
Arif, Z., Munandar, R., Rohaeti, E., Rafi, M. 2023. Detection of Hexavalent Chromium Ion in Water by Optode Membrane. Indones. J. Chem. Stud. 2(2). 76-82. doi: https://doi.org/10.55749/ijcs.v2i2.39.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.