Yellow-Flare Performance Improvement of PVC Addition into Mg-Sodium Nitrate-Based Pyrotechics
DOI:
https://doi.org/10.55749/ijcs.v3i2.60Keywords:
Additives, Burn rate, Emission spectrum, Luminosity, PyrotechnicsAbstract
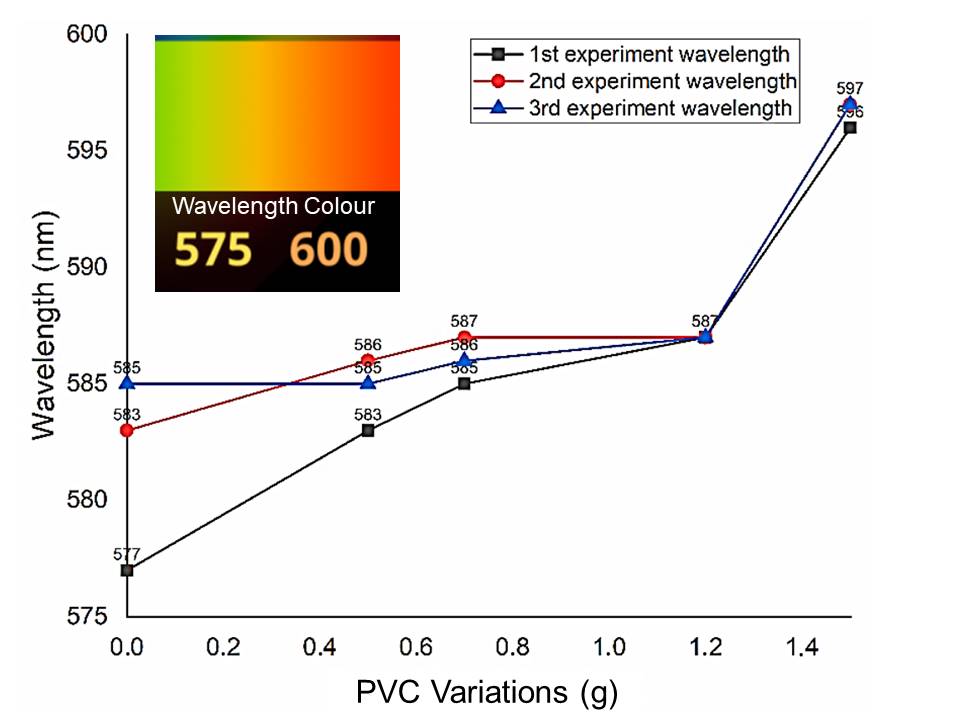
Light pyrotechnics is one strategic defence equipment for civil and military purposes. Additives act as one of the factors that affect the flame in pyrotechnics. Additives were used to slow down the combustion rate so that the flare could burn for a long time without drastically reducing the flame performance of the flare. This study focused on the performance of pyrotechnic flames with variations of PVC as a density-increasing material because it was in the form of a polymer and had high-chlorine content, resulting in a mixture that is difficult to burn. The experiment results exhibited that pyrotechnics without PVC showed intensity with an emission spectrum of 577-585 nm, light intensity of 723-1184 lux, and burning rate of 3.22-3.31 g/s. Increasing the PVC additive composition to 1.5 gr showed emissions with a wavelength of 596-597 nm, decreased intensity from 91-183 lux, and a slower burning rate of 0.72-0.88 g/s. The use of PVC was effectively applied in the 1.76-10.21% fraction and was actively able to slow down the rate of combustion of pyrotechnic mixtures. Hence, PVC could slow down the burning rate and increase density. Adding PVC in yellow pyrotechnics would slow down the burning rate of the pyrotechnic sample with the side effect of reducing the brightness of the yellow color and the intensity of the light.
References
Ambekar A., Kim M., Lee W.H., & Yoh J.J. 2017. Characterization of display pyrotechnic propellants: Burning rate. Appl. Therm. Eng. 121. 761–767. doi: https://doi.org/10.1016/j.applthermaleng.2017.04.097.
Conkling J.A. & Mocella C.J. 2019. Chemistry of Pyrotechnics: Basic Principles and Theory, Third Edition. doi: https://doi.org/10.1201/9780429262135.
Sadek R., Kassem M., Abdo M., & Elbasuney S. 2017. Novel yellow colored flame compositions with superior spectral performance. Def. Technol. 13(1). 33–39. doi: https://doi.org/10.1016/j.dt.2016.12.001.
Moretti J.D., Sabatini J.J., & Poret J.C. 2014. High-performing red-light-emitting pyrotechnic illuminants through the use of perchlorate-free materials. Chem.-A Eur. J. 20(28). 8800–8804. doi: https://doi.org/10.1002/chem.201402654.
Huang Z., Xu S., Chen X., Wang M., Yuan H., Zhao H., & Zhang Y. 2021. Construction of a novel barium-free green-light emitting illuminant based on boron carbide. Propellants, Explos. Pyrotech. 46(9). 1480–1488. doi: https://doi.org/10.1002/prep.202100038.
Ambekar A., Kim M., & Yoh J.J. 2017. Characterization of display pyrotechnic propellants: Colored light. Appl. Therm. Eng. 110. 1066–1074. doi: https://doi.org/10.1016/j.applthermaleng.2016.09.040.
Juknelevicius D., Karvinen E., Klapötke T.M., Kubilius R., Ramanavicius A., & Rusan M. 2015. Copper(I) bromide: an alternative emitter for blue-colored flame pyrotechnics. Chem.-A Eur. J. 21(43). 15354–15359. doi: https://doi.org/10.1002/chem.201502752.
Sabatini J.J. 2018. A review of illuminating pyrotechnics. Propellants, Explos. Pyrotech. 43(1). 28–37. doi: https://doi.org/10.1002/prep.201700189.
Blair L. 2015. Mixing and characterisation of multi-component materials for pyrotechnic applications. Doctoral Disertation. University of Southampton.
Biegańska J. & Barański K. 2022. Experiments with pyrotechnic compositions based on a mathematical model: part II pyrotechnic compositions producing an acoustic effect with optimum properties. Energies. 15(3). 794. doi: https://doi.org/10.3390/en15030794.
Keller F. & Schragen C. 2021. Determination of particulate matter emission factors of common pyrotechnic articles. Propellants, Explos. Pyrotech. 46(5). 825–842. doi: https://doi.org/10.1002/prep.202000292.
Wang F., Pan S., Zhang P., Fan H., Chen Y., & Yan J. 2018. Synthesis and application of phosphorus-containing flame retardant plasticizer for polyvinyl chloride. Fibers Polym. 19(5). 1057–1063. doi: https://doi.org/10.1007/s12221-018-7493-8.
Dilger J.M., Miklaszewski E.J., Papenmeier D.M., Thoreson K.M., Fedick P.W., Coleman J.E., & Bohrer B.C. 2018. Pyrolysis/GC/MS as a method to rapidly profile pyrotechnic formulations for objectionable gaseous emissions. ACS Sustain. Chem. Eng. 6(12). 16990–16999. doi: https://doi.org/10.1021/acssuschemeng.8b04342.
Singh H., Somayajulu M.R., & Bhaskara Rao R. 1989. A study on combustion behavior of magnesiumsodium nitrate binary mixtures. Combust. Flame. 76(1). 57–61. doi: https://doi.org/10.1016/0010-2180(89)90077-1.
Lloret A.T., Sendra S., Lloret J., Louis Cereceda M., & Alba J. 2017. Impact of pyrotechnics over the architectonic heritage. J. Sensors. 214975. doi: https://doi.org/10.1155/2017/7214975.
Sabatini J.J., Nagori A. V., Latalladi E.A., Poret J.C., Chen G., Damavarapu R., & Klapötke T.M. 2011. Applications of high-nitrogen energetics in pyrotechnics: Development of perchlorate-free red star M126A1 hand-held signal formulations with superior luminous intensities and burn times. Propellants, Explos. Pyrotech. 36(4). 373–378. doi: https://doi.org/10.1002/prep.201100023.
Sabatini J.J., Koch E.C., Poret J.C., Moretti J.D., & Harbol S.M. 2015. Chlorine-free red-burning pyrotechnics. Angew. Chemie - Int. Ed. 54(37). 10968–10970. doi: https://doi.org/10.1002/anie.201505829.
Moretti J.D., Sabatini J.J., Poret J.C., & Gilbert R.A. 2015. Development of sustainable, epoxy-bound mg/nano3 compositions for the u.s. army’s 40 mm yellow illuminant flares. ACS Sustain. Chem. Eng. 3(9). 2232–2236. doi: https://doi.org/10.1021/acssuschemeng.5b00508.
Toader G., Rotariu T., Rusen E., Tartiere J., Esanu S., & Zecheru T. 2017. New solvent-free polyurea binder for plastic pyrotechnic compositions. Mater. Plast. 54(1). 22-28. doi: https://doi.org/10.37358/MP.17.1.4777.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Indonesian Journal of Chemical Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.