Synthesis of Fe₃O₄ using the Co-precipitation Method with Temperature and Time Treatment as Methylene Blue Adsorbent
DOI:
https://doi.org/10.55749/ss.v1i2.94Keywords:
Co-precipitation, Magnetite (Fe₃O₄), Magnetic properties, Nanoparticles, Synthesis parametersAbstract
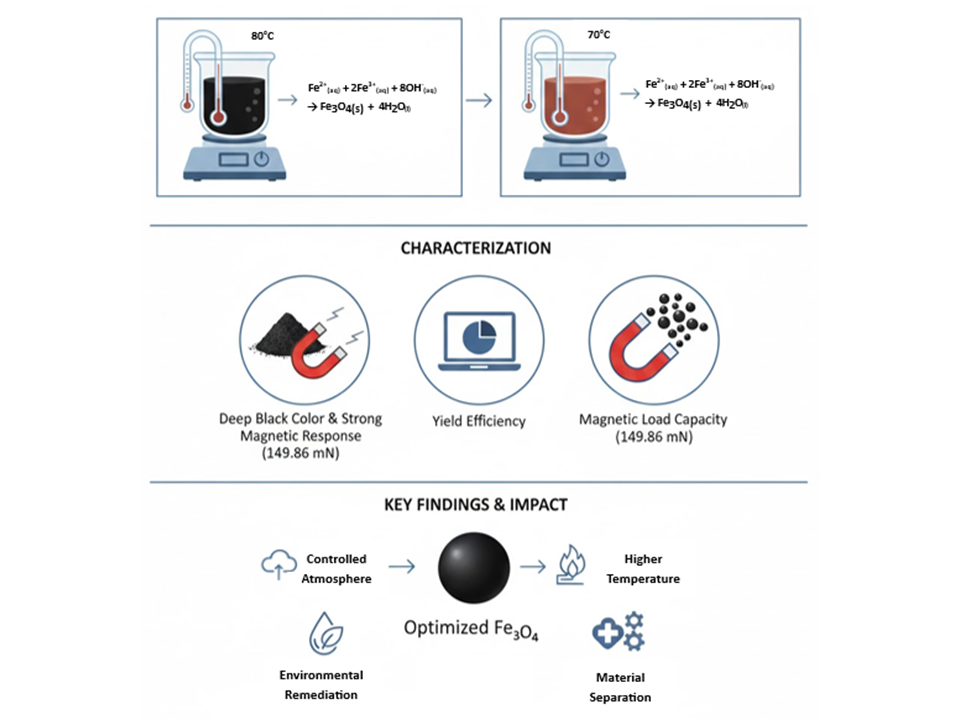
Magnetite nanoparticles (Fe₃O₄) possess unique magnetic properties and are widely applied in various fields such as biomedical technology, environmental remediation, and material separation. This study reports the synthesis of Fe₃O₄ using the co-precipitation method under varying conditions of temperature, reaction time, and atmospheric exposure (open vs. closed system). Ferric and ferrous salts were reacted with ammonium hydroxide under controlled heating at 70°C and 80°C for 60 minutes. The synthesized materials were evaluated through visual color inspection, qualitative magnetic response, yield efficiency, and magnetic load-bearing capacity. The results showed that a closed system at 80°C produced the most optimal Fe₃O₄, indicated by a deep black color, strong magnetic attraction (149.86 mN), and a yield of 92.5%. Comparatively, open systems led to partial oxidation of Fe², resulting in less magnetic phases like maghemite or hematite. The findings confirm that controlling synthesis parameters, especially atmospheric exposure and temperature, significantly influences the purity, particle uniformity, and magnetic strength of Fe₃O₄ nanoparticles, highlighting the importance of optimized synthesis for practical applications.
References
[1] Sinurat, M., Gusti, D.R., Deswardani, F, Safitri, & Sudibyo. 2021. Synthesis and characterization of magnetite (Fe₃O₄) nanoparticles from batanghari river iron sand, Jambi, encapsulated with silica. Tadulako Online Physics Education Journal. 9(1). 106–114.
[2] Agnesistia, R., Narsito, N., & Suyanta, S. 2014. Magnetite-modified bentonite and its application as an Hg(II) adsorbent. Thesis. Department of Chemistry, Universitas Gadjah Mada.
[3] Koo, K.N., Ismail, A.F., Othman, M.H.D., Rahman, M.A., & Zhong, S.T. 2019. Preparation and characterization of superparamagnetic magnetite (Fe₃O₄) nanoparticles: a short review. Mal. J. Fund. Appl. Sci. 15(1). 23–31. https://doi.org/10.11113/mjfias.v15n1.1173
[4] Bustamante-Torres, M., Romero-Fierro, D., Estrella-Núñez, J., Arcentales-Vera, B., Chichande- Proaño, E., & Bucio, E. 2022. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers. 14(4). 752. https://doi.org/10.3390/polym14040752
[5] Eskandari, M.J., & Hasanzadeh, I. 2021. Size-controlled synthesis of Fe₃O₄ Magnetic Nanoparticles via an Alternating Magnetic Field and ultrasonic-assisted chemical co-precipitation. Mater. Sci. Eng. B. 266. 115050. https://doi.org/10.1016/j.mseb.2021.115050
[6] Kustomo, Santosa, S.J., Haarstrick, A. and Li, S.F.Y. 2024. Adsorption of methylene blue onto magnetic nanoparticles and magnetite nanoparticles coated with humic acid. AIP Conf. Proc. 3027(1). 050011. https://doi.org/10.1063/5.0204810
[7] Nguyen, M.D., Tran, H.V., Xu, S., & Lee, T.R. 2021. Fe₃O₄ nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 11(23). 11301. https://doi.org/10.3390/app112311301
[8] Astuti, A., Arief, S., Usna, S.R.A., & Khaira, I. 2022. Synthesis and characterization of structure and magnetic properties of Fe₃O₄@PEG:ZnO nanocomposites. Indonesian Journal of Applied Physics. 12(2). 217–224. https://doi.org/10.13057/ijap.v12n2.58514
[9] Sari, E.O., Fadli, A., & Amri, A. 2019. Effect of temperature and reaction time on the formation of magnetite (Fe₃O₄) nanoparticles by hydrothermal method. Journal Sains dan Teknologi. 18(1). 8–13.
[10] Basuki, R., Apriliyanto, Y.B., Stiawan, E., Pradipta, A.R., Rusdiarso, B. and Putra, B.R. 2025. Magnetic hybrid chitin-horse manure humic acid for optimized Cd(II) and Pb(II) adsorption from aquatic environment. Case Stud. Chem. Environ. Eng. 11. 101138. https://doi.org/10.1016/j.cscee.2025.101138
[11] Arif, Z., Ismawati, I., Hikmah, A.N. and Saprudin, D. 2023. Hydrothermal method to synthesize nanomagnetite by water extract of averrhoa bilimbi: effect of time and reactor size. Indones. J. Chem. Stud. 2(1). 27-34. https://doi.org/10.55749/ijcs.v2i1.29
[12] Rosnati, S.D., & Paryanti, D. 2015. Effect of temperature on the particle size of Fe₃O₄ using PEG-2000 template by co-precipitation method. Jurnal Ilmu Fisika. 7(1). 39–44. https://doi.org/10.25077/jif.7.1.39-44.2015
[13] Pradipta, A.R., Putri, R.A.W., Khairani, I.Y. and Hasnowo, L.A., 2024. Magnetically Chitosan-Silica-Based Biosorbent as Efficient Removal of Au (III) in Artificial Wastewater. Indones. J. Chem. Stud. 3(1). 8-15. https://doi.org/10.55749/ijcs.v3i1.40
[14] Insin, N. 2020. Magnetic Nanocarriers of Bioactives: Structural and Delivery System Designs. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents. pp. 211–242. Academic Press. https://doi.org/10.1016/B978-0-12-819666-3.00007-9
[15] Marlinda, L., Malikhah, W., Ishartono, B. and Basuki, R. 2023. Magnetically separable humic acid-chitin based adsorbent as Pb(II) uptake in synthetic wastewater. Indones. J. Chem. Stud. 2(1). 13-21. https://doi.org/10.55749/ijcs.v2i1.22
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sorption Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









