Effect of Different Temperatures in Magnetite Synthesis on Methylene Blue Adsorption
DOI:
https://doi.org/10.55749/ss.v1i2.84Keywords:
Ammonia, Close system, Coprecipitation, Magnetite, Methylene blue, Open systemAbstract
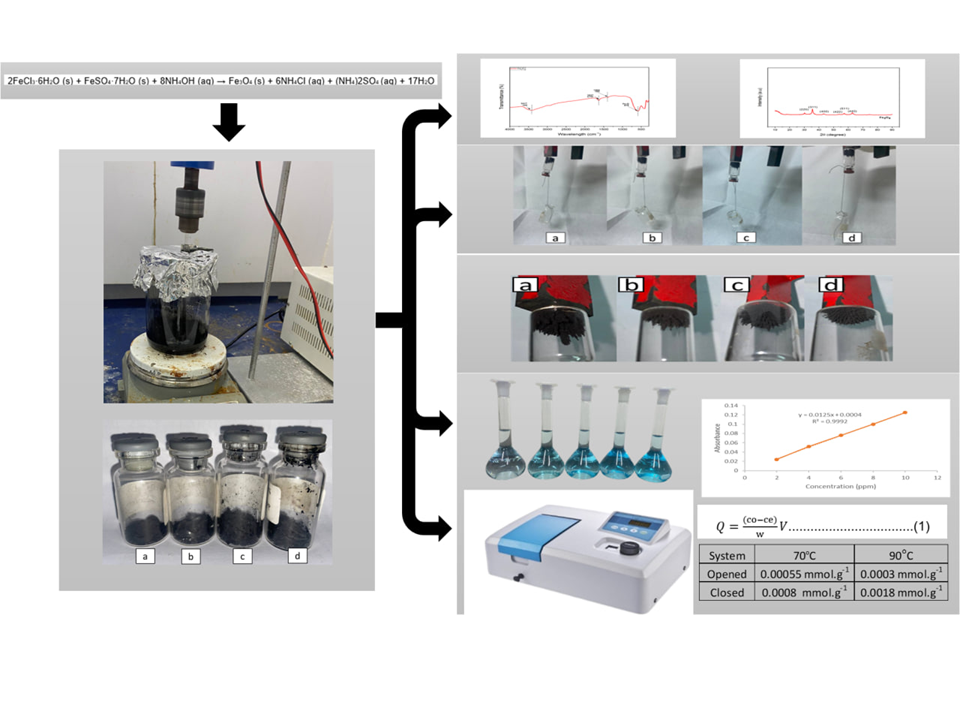
This study aims to synthesize magnetite (Fe₃O₄) particles using the coprecipitation method, with variations in temperature (70°C and 90°C) and reaction system (open and closed) to evaluate their effects on product quality. Characterization was conducted using FTIR, XRD, and organoleptic observation to confirm the formation of Fe₃O₄. Additional tests included magnetic attraction measurements through mass response and adsorption capacity (Q) analysis using methylene blue. FTIR analysis showed absorption bands at 3417.00 cm⁻¹, 1627 cm⁻¹, 1404 cm⁻¹, and 578 cm⁻¹, indicating the presence of O–H, C=O, and Fe–O functional groups. XRD patterns revealed diffraction peaks at 2θ values of 30.27°, 35.23°, 43.22°, 53.71°, 57.43°, and 62.11°, confirming the spinel crystal structure of Fe₃O₄. The sample synthesized at 90°C under closed conditions exhibited a darker black color and higher mass yield, suggesting improved crystallinity and phase purity. The closed system also showed higher adsorption capacities of 0.0008 mmol·g⁻¹ at 70°C and 0.0018 mmol·g⁻¹ at 90°C, along with stronger magnetic response. The open system produced a black precipitate with lower yield and weaker magnetic response, suggesting oxidation of Fe²⁺ to Fe³⁺ due to direct contact with oxygen, leading to the formation of compounds such as hematite or maghemite with lower magnetic properties. These results confirm that higher reaction temperatures and closed conditions optimally enhance the quality and stability of magnetite.
References
[1] Santos, L.G.D., Buelvas, D.D.A., Valezi, D.F., Vicentin, B.L.S., Rocha, C.M.M., Mauro, E.D, & Porta, F., De A.L. 2025. Microstructural and magnetic properties of polyamide-based recycled composites with iron oxide nanoparticles. Magnetism. 5(1).1–16. https://doi.org/10.3390/magnetism5010005
[2] Duglet, R., Sharma, D., Singh, V., Sharma, D., & Singh, M. 2025. Temperature-driven evolution of hematite (α-Fe2O3) nanoparticles: a study on structural, morphological and magnetic properties. Solid State Commun. 396. 115761. https://doi.org/10.1016/j.ssc.2024.115761
[3] Dhahri, R., Benamara, M., Bouzidi, S., Ben Moussa, S., Alzahrani, A.Y.A., Nassar, K.I., Zahmouli, N., Elkenany, E.B., & Al-Syadi, A.M. 2024. Effect of Gd doping on the microstructure and electrical characteristics of maghemite (Γ-Fe₂O₃) ceramics. J. Sol-Gel Sci. Technol. 113. 225–242. https://doi.org/10.1007/s10971-024-06598-0
[4] Chowdhury, M.Z.B., Islam, M.T., Alqahtani, A., Alshammari, A. S., Soliman, M.S., Alamri, S., & Samsuzzaman, M. 2024. Synthesis and characterization of Mn–Zn ferrite-based flexible penta-band metamaterial for sensing applications. Opt. Laser Technol. 175. 110744.
https://doi.org/10.1016/j.optlastec.2024.110744
[5] Lin, C.C., Wu, Y.C. and Wu, K.Y. 2024. Feasibility of using chemical co-precipitation and a high-gravity reactor with blade packings for continuous production of magnetite nanoparticles. J. Taiwan Inst. Chem. E. 162. 105620.
https://doi.org/https://doi.org/10.1016/j.jtice.2024.105620
[6] Zulfikar, T.M. and Viena, V. 2024. March. Application of sol-gel method and co-precipitation in the material synthesis process of magnetite fe3o4 nanoparticles. In Psroceeding of International Conference on Multidisciplinary Research. 6(2). 311-316.
[7] Ognjanović, M., Bošković, M., Kolev, H., Dojčinović, B., Vranješ-Đurić, S., & Antić, B. 2024. Synthesis, surface modification and magnetic properties analysis of heat-generating cobalt-substituted magnetite nanoparticles. Nanomaterials. 14(9). 782. https://doi.org/10.3390/nano14090782
[8] Kustomo, K. 2020. Uji Karakterisasi dan mapping magnetit nanopartikel terlapisi asam humat dengan Scanning-Electron-Microscope-Energy Dispersive X-Ray (SEM-EDX). Indonesian Journal of Chemical Science. 9(3). 148-153.
[9] Saragi, T., Permana, B., Saputri, M., Safriani, L., Rahayu, I., & Risdiana, R. 2018. Sintesis nanopartikel magnetik dengan metode kopresipitasi. Jurnal Material dan Energi Indonesia. 7(2). 17. https://doi.org/10.24198/jmei.v7i02.15393 s
[10] Nkurikiyimfura, I., Wang, Y., Safari, B., & Nshingabigwi, E. 2020. Temperature-dependent magnetic properties of magnetite nanoparticles synthesized via coprecipitation method. J. Alloys Compd. 846. 156344.
https://doi.org/10.1016/j.jallcom.2020.156344
[11] Kumar, P., Khanduri, H., Pathak, S., Singh, A., Basheed, G.A., & Pant, R.P. 2020. Temperature selectivity for single phase hydrothermal synthesis of peg-400 coated magnetite nanoparticles. Dalton Trans. 49(25). 8672–8683. https://doi.org/10.1039/d0dt01318h
[12] Cursaru, L.M., Piticescu, R.M., Dragut, D.V., Morel, R., Thébault, C., Carrière, M., Joisten, H., & Dieny, B. 2020. One-step soft chemical synthesis of magnetite nanoparticles under inert gas atmosphere: magnetic properties and in vitro study. Nanomaterials. 10(8). 1500.
https://doi.org/10.3390/nano10081500
[13] Ngatijo, N., Gusti, D., Fadhilah, A. and Khairunnisah, R. 2020. Adsorben magnetit terlapis dimerkaptosilika untuk adsorpsi anion logam [AuCl4]- dan [Cr2O7]-. Jurnal Riset Kimia. 11(2). 113-120. http://dx.doi.org/10.1016/j.jmmm.2020.166408
[14] Sani, S., Adnan, R., & Mohamed Iqbal, M.A. 2021. One-step statistical design of experiment for the screening and optimization of magnetite nanoparticles yields from solvothermal synthesis. Microporous And Mesoporous Mater. 312. 110775. https://doi.org/10.1016/j.micromeso.2020.110775
[15] Dubey, V., & Kain, V. 2018. Synthesis of magnetite by coprecipitation and sintering and its characterization. Mater. Manuf. Process. 33(8). 835–839.
https://doi.org/10.1080/10426914.2017.1401720
[16] Novoselova, L.Y. 2021. Nanoscale magnetite: new synthesis approach, structure and properties. Appl. Surf. Sci. 539. 148275. https://doi.org/10.1016/j.apsusc.2020.148275 ss
[17] Mashkoor, F., & Nasar, A. 2020. Magsorbents: potential candidates in wastewater treatment technology–a review on the removal of methylene blue dye. J. Magn. Magn. Mater. 500. 166408. https://doi.org/10.1016/j.jmmm.2020.166408
[18] Nitsae, M., Solle, H.R., Martinus, S.M., & Emola, I.J. 2021. Studi adsorpsi metilen biru menggunakan arang aktif tempurung lontar (Borassus flabellifer l.) asal nusa tenggara timur. Jurnal Kimia Riset. 6(1). 46-57.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sorption Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









