Synthesis of Magnetite/Chitin/Fulvic Acid Derived from Goat Manure Compost and Adsorption Study of Zn(II) for Water Security Enhancement
DOI:
https://doi.org/10.55749/ss.v1i1.82Keywords:
Adsorption, Fulvic acid, Goat manure compost, Magnetite/Chitin/FA, Zn(II)Abstract
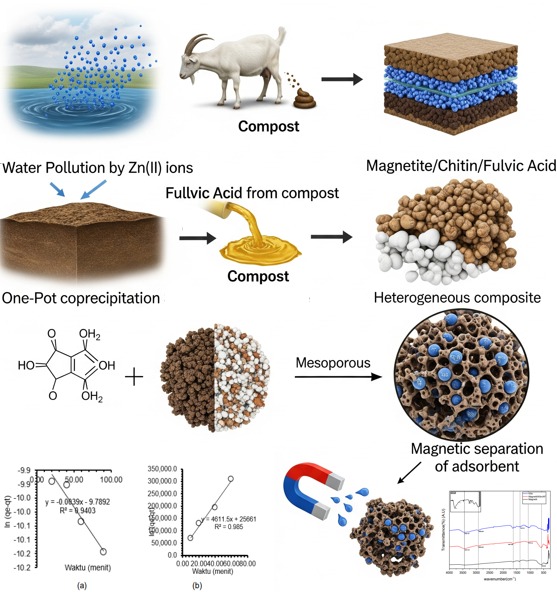
Water pollution due to heavy metals such as Zn(II) poses a risk to the environment and health. This study aims to synthesize Magnetite/Chitin/Fulvic Acid (AF)-based composite adsorbent from goat feces compost and evaluate its effectiveness in adsorbing Zn(II) ions. Fulvic acid was extracted through alkaline-acid method and synthesized together with chitin and magnetite using one pot coprecipitation method. Characterization using FTIR, XRD, and BET showed successful synthesis with mesoporous structure for BET (average pore size 6.15 nm, surface area 41.77 m²/g). Isotherm studies showed that the adsorption of Zn(II) showed a good fit with the Freundlich (R² = 0.9967) and Temkin (R² = 0.9968) models, indicating multilayer adsorption on the heterogeneous surface. The composite also shows good adsorption ability and can be magnetically separated, making it an environmentally friendly and efficient potential adsorbent for wastewater treatment applications.
References
[1] Basuki, R., Santosa, S.J., & Rusdiarso, B. 2017. Ekstraksi adsorben ramah lingkungan dari matriks biologi: asam humat tinja kuda (AH-TK). Chempublish Journal. 2(1). 13-25.
[2] Wathoni, A.Z., Pratiwi, A.I., & Suci, F.C. 2021. Penurunan kadar logam berat nikel limbah cair industri pada pengolahan air limbah industri di karawang. Journal of Industrial Process and Chemical Engineering (JOICHE). 1(2). 40-45. Doi: https://doi.org/10.31284/j.joiche.2021.v1i2.2440
[3] Widayatno, T. 2017. Adsorpsi logam berat (Pb) dari limbah cair dengan adsorben arang bambu aktif. Jurnal teknologi bahan alam. 1(1). 17-23.
[4] Nurdila, F.A., Asri, N.S. & Suharyadi, E. 2015. Adsorpsi logam Tembaga (Cu), Besi (Fe), dan Nikel (Ni) dalam limbah cair buatan menggunakan nanopartikel cobalt ferrite (CoFe2O4) Jurnal Fisika Indonesia. 19(55). 23-27. Doi: https://doi.org/10.22146/jfi.24368
[5] Ngatijo, N., Bemis, R., & Ihsan, M. 2021. Nanofikasi fraksi tanah gambut untuk modifikator nanomagnetit/ah-kitosan sebagai kandidat penanggulangan pencemaran zat warna. Chempublish Journal. 5(2). 140-150. Doi: https://doi.org/10.22437/chp.v5i2.11105
[6] Ukalska-Jaruga, A., Bejger, R., Debaene, G. & Smreczak, B. 2021. Characterization of soil organic matter individual fractions (fulvic acids, humic acids, and humins) by spectroscopic and electrochemical techniques in agricultural soils. Agronomy.11(6). 1067. Doi: https://doi.org/10.3390/agronomy11061067
[7] Zhang, Y., Qin, X., An, J. & Zu, B. 2025. Adsorption and reduction of Cr(VI): mechanistic investigations of magnetite-fulvic acid complexes. Environ. Technol. 1-13. Doi: https://doi.org/10.1080/09593330.2025.2546122
[8] Hidayah, M., Kustomo, K., & Yunita, A.N.I. 2022. Batch adsorption of Pb(II) batch using humic acid from goat dung. al Kimiya: Jurnal Ilmu Kimia dan Terapan. 9(2). 55-61. https://doi.org/10.15575/ak.v9i2.19735
[9] Luo, S., Zhen, Z., Zhu, X., Ren, L., Wu, W., Zhang, W., Chen, Y., Zhang, D., Song, Z., Lin, Z. and Liang, Y.Q. 2021. Accelerated atrazine degradation and altered metabolic pathways in goat manure assisted soil bioremediation. Ecotoxicol. Environ. Saf. 221. 112432. Doi: https://doi.org/10.1016/j.ecoenv.2021.112432
[10] Shahib, I.I., Ifthikar, J., Wang, S., Elkhlifi, Z., He, L. & Chen, Z., 2023. Elimination of hazardous Se(IV) through adsorption-coupled reduction by iron nanoparticles embedded on mesopores of chitin obtained from waste shrimp shells. Environmental Science and Pollution Research. 30(57). 119961-119973. Doi: https://doi.org/10.1007/s11356-023-30743-x
[11] Rahaman, M.H., Islam, M.A., Islam, M.M., Rahman, M.A., & Alam, S.N. 2021. Biodegradable composite adsorbent of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Current Research in Green and Sustainable Chemistry. 4. 100119. https://doi.org/10.1016/j.crgsc.2021.100119
[12] Shi, J., Li, H., Lu, H., & Zhao, X. 2015. Use of carboxyl functional magnetite nanoparticles as potential sorbents for the removal of heavy metal ions from aqueous solution. J. Chem. Eng. Data. 60(7). 2035-2041. Doi: https://doi.org/10.1021/je5011196
[13] Amjadi, M., Samadi, A., & Manzoori, J.L. 2015. A composite prepared from halloysite nanotubes and magnetite (Fe3O4) as a new magnetic sorbent for the preconcentration of cadmium (II) prior to its determination by flame atomic absorption spectrometry. Mikrochim. Acta. 182. 1627-1633. Doi: https://doi.org/10.1007/s00604-015-1491-y
[14] Ngatijo, Marlinda, L., Malikhah, W., Ishartono, B., & Basuki, R. 2023. Magnetically separable humic acid-chitin based adsorbent as Pb(II) uptake in synthetic wastewater. Indones. J. Chem. Stud. 2(1). 13-21. Doi: https://doi.org/10.55749/ijcs.v2i1.22
[15] Zhang, P., Zhang, H., Wu, G., Chen, X., Gruda, N., Li, X., Dong, J. & Duan, Z. 2021. Dose-dependent application of straw-derived fulvic acid on yield and quality of tomato plants grown in a greenhouse. Front. Plant Sci. 12. 736613. Doi: https://doi.org/10.3389/fpls.2021.736613
[16] Zavarzina, A.G., Danchenko, N.N., Demin, V.V., Artemyeva, Z.S. and Kogut, B.M. 2021. Humic substances: hypotheses and reality (a review). Eurasian Soil Sci. 54(12). 1826-1854. Doi: https://doi.org/10.1134/S1064229321120164
[17] Yuan, L., Yan, M., Huang, Z., He, K., Zeng, G., Chen, A., Hu, L., Li, H., Peng, M., Huang, T. and Chen, G. 2019. Influences of pH and metal ions on the interactions of oxytetracycline onto nano-hydroxyapatite and their co-adsorption behavior in aqueous solution. J. Colloid Interface Sci. 541. 101-113. Doi: https://doi.org/10.1016/j.jcis.2019.01.078
[18] Basuki, R., Apriliyanto, Y.B., Stiawan, E., Pradipta, A.R., Rusdiarso, B., & Putra, B.R. 2025. Magnetic hybrid chitin-horse manure humic acid for optimized Cd(II) and Pb(II) adsorption from aquatic environment. Case Stud. Chem. Environ. Eng. 11. 101138. Doi: https://doi.org/10.1016/j.cscee.2025.101138
[19] Krisbiantoro, P.A., Santosa, S.J., & Kunarti, E.S. 2017. Synthesis of fulvic acid-coated magnetite (Fe3O4-FA) and its application for the reductive adsorption of [AuCl4]–. Indones. J. Chem. 17(3). 453-460. Doi: http://dx.doi.org/10.22146/ijc.24828
[20] Elsayed, S.A., El-Sayed, I.E., & Tony, M.A. 2022. Impregnated chitin biopolymer with magnetic nanoparticles to immobilize dye from aqueous media as a simple, rapid and efficient composite photocatalyst. Appl. Water Sci. 12(11). 252. Doi: https://doi.org/10.1007/s13201-022-01776-3
[21] Samoilova, N.A. and Krayukhina, M.A. 2020. Chitin-based magnetic composite for the removal of contaminating substances from aqueous media. Russ. Chem. Bull. 69(6).1157-1164. Doi: https://doi.org/10.1007/s11172-020-2883-7
[22] Salam, M.A. 2017. Preparation and characterization of chitin/magnetite/multiwalled carbon nanotubes magnetic nanocomposite for toxic hexavalent chromium removal from solution. J. Mol. Liq. 233. 197-202. Doi: https://doi.org/10.1016/j.molliq.2017.03.023
[23] Stevenson F.J. 1994. Humus Chemistry: Genesys, Composition, Reaction, 2nd edition. John Wiley & Sons: New York.
[24] Gunzler, H. and Gremlich, H.U., 2002. Qualitative spectral interpretation. IR spectroscopy: An introduction. 171-274. Wiley-VCH: Weinheim, Germany.
[25] Chander, S., Yadav, S., Sharma, H.R. and Gupta, A. 2024. Sequestration of Cd(II) utilizing biowaste-fabricated recyclable mesoporous magnetite (Fe3O4) nano-adsorbent: Process optimization, thermodynamic investigation, simulation modeling, and feasibility for electroplating effluent. J. Alloys Compd. 986. 174088. Doi: https://doi.org/10.1016/j.jallcom.2024.174088
[26] Zarghani, M. & Akhlaghinia, B. 2016. Magnetically separable chitin as an eco-friendly nanocatalyst with high efficiency for green synthesis of 5-substituted-1H-tetrazoles under solvent-free conditions. RSC Adv. 6(38). 31850-31860. https://doi.org/10.1039/C6RA07252F
[27] Jiang, Y., Cai, D., Liu, Q., Shi, Q., Wang. 2023. Adsorption properties and mechanism of Suaeda biochar and modified materials for tetracycline. Environ. Res. 235. 116549. Doi: https://doi.org/10.1016/j.envres.2023.116549
[28] Kusuma, A.K.K.W., Harjito, H., & Jumaeri, J. 2018. Perbandingan massa Ca(NO3)2 dengan SBA-15 terhadap kebasaan katalis reaksi gliserolisis. Indonesian Journal of Chemical Science. 7(2). 175-181.
[29] Pylypchuk, I.V., Kołodyńska, D., Kozioł, M., & Gorbyk, P.P. 2016. Gd-DTPA adsorption on chitosan/magnetite nanocomposites. Nanoscale Res. Lett. 11. 1-10. Doi: https://doi.org/10.1186/s11671-016-1363-3
[30] Serafin, J., & Dziejarski, B. 2023. Application of isotherms models and error functions in activated carbon CO2 sorption processes. Micropor. Mesopor. Mat. 354. 112513. Doi: https://doi.org/10.1016/j.micromeso.2023.112513
[31] Turlapati, B.K. Prusty, S.K. Pal, E. Raja. 2023. Examining supercritical methane adsorption on nanoporous shales using constant and varying adsorbed phase density approaches. Energy Fuels. 37(3). 2078–2090. Doi: https://doi.org/10.1021/acs.energyfuels.2c03922
[32] Shoaib, A.G., Ragab, S., El Sikaily, A., Yılmaz, M., & El Nemr, A. 2023. Thermodynamic, kinetic, and isotherm studies of Direct Blue 86 dye absorption by cellulose hydrogel. Scientific Reports. 13(1). 5910. Doi: https://doi.org/10.1038/s41598-023-33078-2
[33] Charpentier, T.V., Neville, A., Lanigan, J.L., Barker, R., Smith, M.J. and Richardson, T. 2016. Preparation of magnetic carboxymethylchitosan nanoparticles for adsorption of heavy metal ions. ACS Omega. 1(1). 77-83. Doi: https://doi.org/10.1021/acsomega.6b00035
[34] Karimi, A.R., Fateh, S., Bayat, F. & Abdollahi, M. 2025. Design and characterization of tunable chitosan hydrogels cross-linked with epiclon through amide bond formation modified with multi-walled carbon nanotubes for adsorption of aflatoxin B1 from aqueous solutions. J. Macromol. Sci., A. 62(3). 263-273. Doi: https://doi.org/10.1080/10601325.2025.2467050
[35] Abdus-Salam, N. & Adekola, S.K. 2018. Adsorption studies of zinc (II) on magnetite, baobab (Adansonia digitata) and magnetite–baobab composite. Appl. Water Sci. 8(8). 222. Doi: https://doi.org/10.1007/s13201-018-0867-7
[36] Zhang, N., Reguyal, F., Praneeth, S., & Sarmah, A. K. 2023. A novel green synthesized magnetic biochar from white tea residue for the removal of Pb(II) and Cd(II) from aqueous solution: Regeneration and sorption mechanism. Environ. Pollut. 330. 121806. Doi: https://doi.org/10.1016/j.envpol.2023.121806
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sorption Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









