Adsorption Ni(II) on Magnetic Fulvic Acid-Chitosan: Kinetics and Isotherm Study
DOI:
https://doi.org/10.55749/ss.v1i1.79Keywords:
Adsorption, Fulvic Acid, Chitosan, Magnetite, Ni(II)Abstract
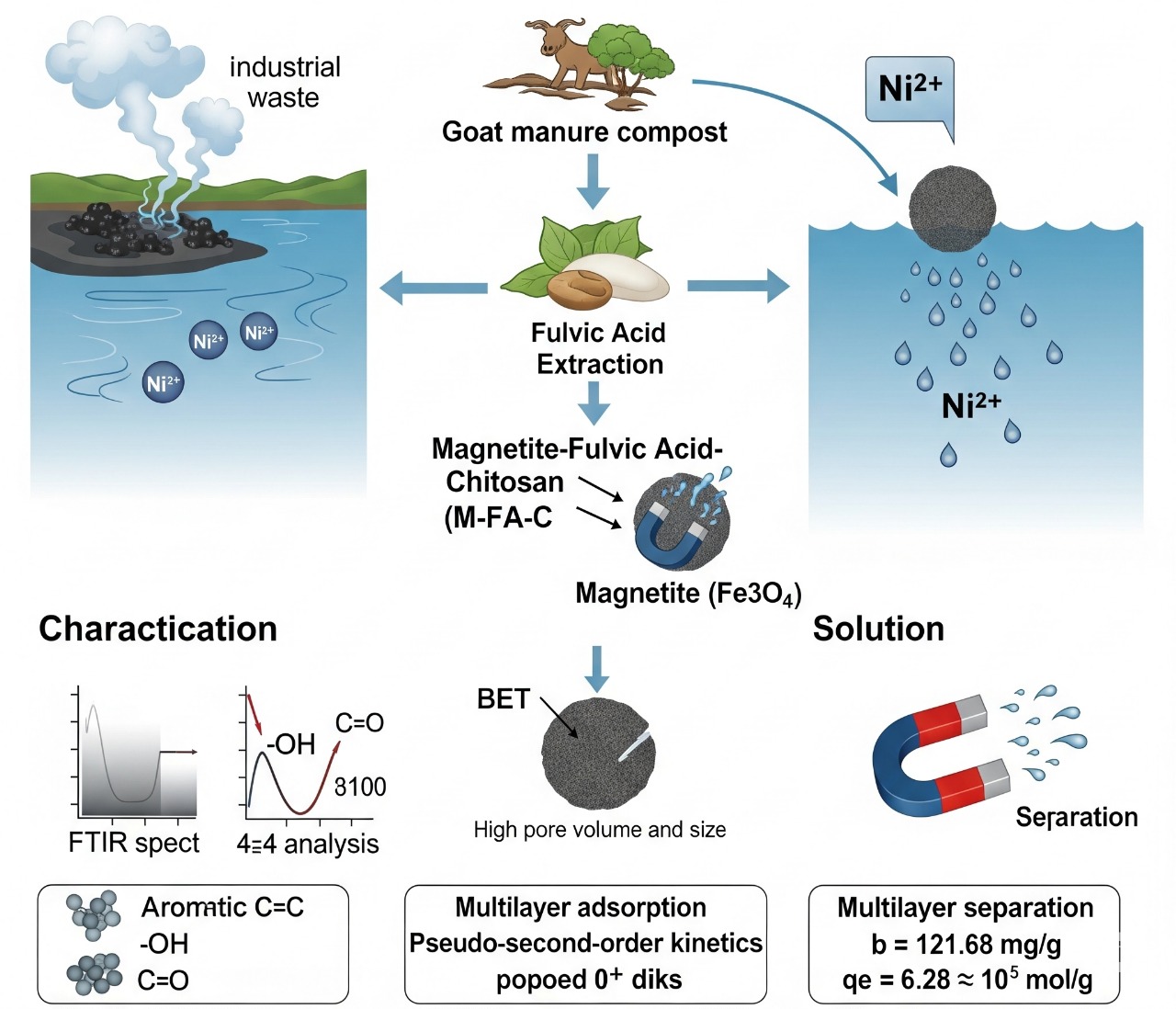
Indonesia, as one of the most populous countries in the world, requires clean water sources. Industrial waste that is improperly discharged pollutes water bodies with hazardous metals. Adsorption is one of the effective methods for reducing the concentration of harmful metals in water. This study utilized fulvic acid extracted from goat manure compost and combined it with chitosan and magnetite as an adsorbent material for Ni(II). The FTIR results for the magnetite-fulvic acid-chitosan composite showed a peak at 1627 cm⁻¹, indicating the presence of aromatic C=C, aromatic ring -OH, and quinone C=O groups, which confirm the binding of fulvic acid. BET analysis was performed on magnetite and magnetite-fulvic acid-chitosan, and the pore volume and pore size were found to be 0.177488 cm³/g and 6.5394 nm, respectively. The composite exhibited magnetic behavior due to the attraction between the magnetite-fulvic acid-chitosan and an external magnet. Adsorption tests using isotherm and kinetic models revealed that Ni(II) adsorption followed a multilayer mechanism and pseudo-second-order kinetics, with a b value of 121.68 mg/g and an experimental qe of 6.28 × 10⁻⁵ mol/g. This shows that the magnetite-fulvic acid-chitosan composite is a promising, sustainable, and magnetically separable adsorbent for the effective removal of nickel ions from contaminated water.
References
[1] Mutoffar, M.M., Naseer, M. and Fadillah, A. 2022. Klasifikasi kualitas air sumur menggunakan algoritma random forest. Naratif: Jurnal Nasional Riset, Aplikasi dan Teknik Informatika. 4(2). 138-146. Doi: https://doi.org/10.53580/naratif.v4i2.160.
[2] Dewi, E.P., Wijaya, A., Sujatini, S., Rahmana, D., Mandela, C. and Gulit, F. 2020. Penerapan Double Skin Facade Pada Daerah Iklim Tropis. IKRA-ITH Teknologi Jurnal Sains dan Teknologi. 4(2).1-7.
[3] Tran, H.V., Dai Tran, L. and Nguyen, T.N. 2010. Preparation of chitosan/magnetite composite beads and their application for removal of Pb(II) and Ni(II) from aqueous solution. Mater. Sci. Eng., C. 30(2). 304-310. Doi: https://doi.org/10.1016/j.msec.2009.11.008.
[4] Anderson, A., Anbarasu, A., Pasupuleti, R.R., Manigandan, S., Praveenkumar, T.R. and Kumar, J.A. 2022. Treatment of heavy metals containing wastewater using biodegradable adsorbents: A review of mechanism and future trends. Chemosphere. 295. 133724. Doi: https://doi.org/10.1016/j.chemosphere.2022.133724.
[5] Carmona, B. and Abejón, R. 2023. Innovative membrane technologies for the treatment of wastewater polluted with heavy metals: perspective of the potential of electrodialysis, membrane distillation, and forward osmosis from a bibliometric analysis. Membranes. 13(4). 385.
Doi: https://doi.org/10.3390/membranes13040385.
[6] Rashid, R., Shafiq, I., Akhter, P., Iqbal, M.J. and Hussain, M. 2021. A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ. Sci. Pollut. Res. 28. 9050-9066. Doi: https://doi.org/10.1007/s11356-021-12395-x.
[7] Chikri, R., Elhadiri, N., Benchanaa, M. and El Maguana, Y. 2020. Efficiency of sawdust as low‐cost adsorbent for dyes removal. J. Chem. 2020(1). 8813420. Doi: https://doi.org/10.1155/2020/8813420.
[8] Ateş, A., Mert, Y. and Timko, M.T. 2023. Evaluation of characteristics of raw tea waste-derived adsorbents for removal of metals from aqueous medium. Biomass Convers. Biorefin. 13. 7811-7826. Doi: https://doi.org/10.1007/s13399-021-01721-5.
[9] Tamjidi, S. and Ameri, A. 2020. A review of the application of sea material shells as low cost and effective bio-adsorbent for removal of heavy metals from wastewater. Environ. Sci. Pollut. Res. 27(25). 31105-31119. Doi: https://doi.org/10.1007/s11356-020-09655-7.
[10] Boguta, P. and Sokołowska, Z. 2020. Zinc binding to fulvic acids: Assessing the impact of pH, metal concentrations and chemical properties of fulvic acids on the mechanism and stability of formed soluble complexes. Molecules. 25(6). 1297. Doi: https://doi.org/10.3390/molecules25061297.
[11] Ewis, D., Mahmud, N., Benamor, A., Ba-Abbad, M.M., Nasser, M. and El-Naas, M. 2022. Enhanced removal of diesel oil using new magnetic bentonite-based adsorbents combined with different carbon sources. Water, Air, & Soil Pollution. 233(6). 195. Doi: https://doi.org/10.1007/s11270-022-05641-6.
[12] Ngatijo, Basuki, R., Rusdiarso, B., Nuryono. 2020. Sorption-desorption profile of Au(III) onto silica modified quaternary amines (SMQA) in gold mining effluent. J. Environ. Chem. Eng. 8(3). 103747. Doi: https://doi.org/10.1016/j.jece.2020.103747.
[13] Yadav, B.S. and Dasgupta, S. 2022. Effect of time, pH, and temperature on kinetics for adsorption of methyl orange dye into the modified nitrate intercalated MgAl LDH adsorbent. Yadav, B.S. and Dasgupta, S., 2022. Effect of time, pH, and temperature on kinetics for adsorption of methyl orange dye into the modified nitrate intercalated MgAl LDH adsorbent. norg. Chem. Commun. 137. 109203. Doi: https://doi.org/10.1016/j.inoche.2022.109203.
[14] Basuki, R., Apriliyanto, Y.B., Stiawan, E., Pradipta, A.R., Rusdiarso, B. and Putra, B.R., 2025. Magnetic hybrid chitin-horse manure humic acid for optimized Cd(II) and Pb(II) adsorption from aquatic environment. Case Stud. Chem. Environ. Eng. 101138. Doi: https://doi.org/10.1016/j.cscee.2025.101138.
[15] da Silva Alves, D.C., Healy, B., Pinto, L.A.D.A., Cadaval Jr, T.R.S.A. and Breslin, C.B. 2021. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules. 26(3). 594. Doi: https://doi.org/10.3390/molecules26030594.
[16] Rana, T. and Roy, A. 2024. Goat manure production and waste management. Trends in Clinical Diseases, Production and Management of Goats. 203-215. Doi: https://doi.org/10.1016/B978-0-443-23696-9.00007-9.
[17] Ayilara, M.S., Olanrewaju, O.S., Babalola, O.O. and Odeyemi, O. 2020. Waste management through composting: Challenges and potentials. Sustainability. 12(11). 4456. Doi: https://doi.org/10.3390/su12114456.
[18] Islam, M.A., Morton, D.W., Johnson, B.B. and Angove, M.J. 2020. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 247. 116949. Doi: https://doi.org/10.1016/j.seppur.2020.116949.
[19] Giraldo, J.D. and Rivas, B.L. 2021. Direct ionization and solubility of chitosan in aqueous solutions with acetic acid. Polymer Bulletin. 78. 1465-1488. Doi: https://doi.org/10.1007/s00289-020-03172-w.
[20] Blowes, D., Ptacek, C., Jambor, J. and Weisener, C. 2005. The geochemistry of acid mine. Environ. Geochem. 9. 149.
[21] Ismail, H., Zainuddin, Z., Mohamad, H. and Hamid, M.A.A., 2022. Compatibility of Concentrated NaOH as a Precipitation Agent in the Synthesis of Maghemite (γ-Fe2O3) Nanoparticles via Co-precipitation Method. J. Phys. Sci. 33(2). 61-75. Doi: https://doi.org/10.21315/jps2022.33.2.4.
[22] Zhuang, J., Li, M., Pu, Y., Ragauskas, A.J. and Yoo, C.G. 2020. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 10(12). 4345. Doi: https://doi.org/10.3390/app10124345.
[23] Gong, G., Xu, L., Zhang, Y., Liu, W., Wang, M., Zhao, Y., Yuan, X. and Li, Y., 2020. Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega. 5(43). 27953-27961. Doi: https://doi.org/10.1021/acsomega.0c03388.
[24] Reyes, M., Herrera, G., Escudero, R., Patiño, F., Reyes, I.A., Flores, M., Palacios, E.G., Juárez, J. and Barrientos, F. 2022. Surface spectroscopy of pyrite obtained during grinding and its magnetisation. Minerals. 12(11). 1444. Doi: https://doi.org/10.3390/min12111444.
[25] Brião, G.D.V., De Andrade, J.R., da Silva, M.G.C. and Vieira, M.G.A. 2020. Removal of toxic metals from water using chitosan-based magnetic adsorbents. A review. Environ. Chem. Lett. 18. 1145-1168. Doi: https://doi.org/10.1007/S10311-020-01003-Y.
[26] Kyriakopoulos, G.L., Tsimnadis, K., Sebos, I. and Charabi, Y., 2024. Investigating the effect of pore size distribution on the sorption types and the adsorption-deformation characteristics of porous continua: the case of adsorption on carbonaceous materials. Crystals. 14(8). 742. Doi: https://doi.org/10.3390/cryst14080742.
[27] Sharifi, M.J., Nouralishahi, A. and Hallajisani, A. 2023. Fe3O4-chitosan nanocomposite as a magnetic biosorbent for removal of nickel and cobalt heavy metals from polluted water. Int. J. Biol. Macromol. 248. 125984. Doi: https://doi.org/10.1016/j.ijbiomac.2023.125984.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sorption Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









