Kinetic Study of Mg(II) Adsorption on Activated Coal Bottom Ash
DOI:
https://doi.org/10.55749/ss.v1i1.77Keywords:
Adsorption, Coal bottom ash, Kinetics, Mg(II) metal ionsAbstract
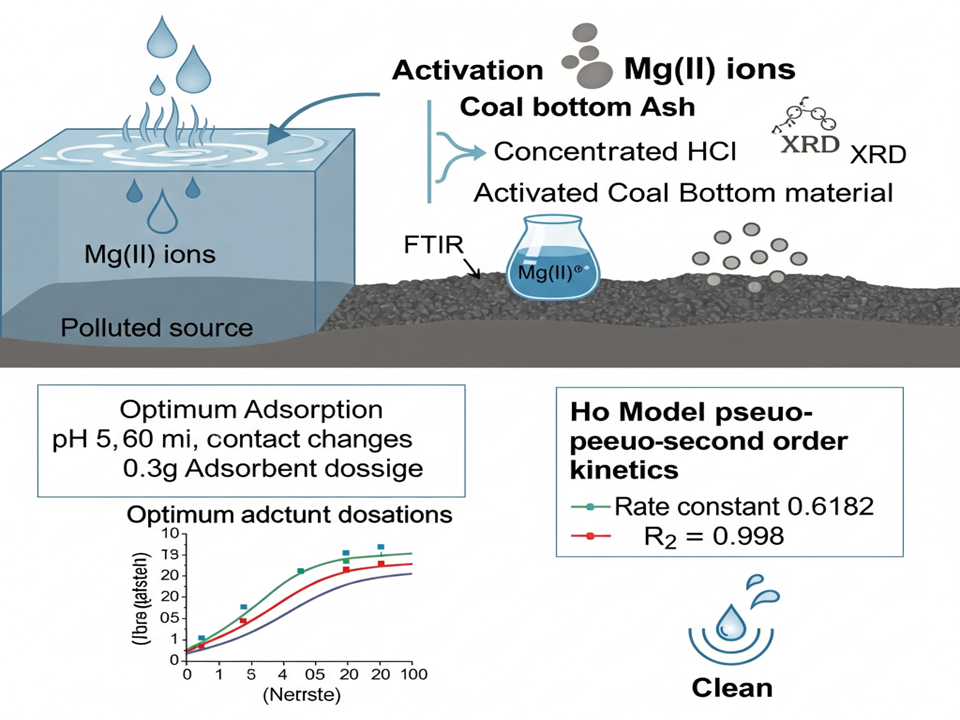
The research of sadsorption of Mg(II) ions on coal bottom ash as adsorbent has been carried out. The research was conducted by activating the coal bottom ash using concentrated HCl. Characterization of activated coal bottom ash was done by using Fourier Transform Infra-Red (FTIR) spectroscopy and X-Ray Difraction (XRD) analysis. Parameters of metal adsorption examined in this study include the effect of pH, mass of adsorbent, and interaction time. The concentration of each metal ion remaining in the solution after adsorption and desorption was determined using atomic absorption spectrophotometer. The result showed that activated coal bottom ash has been carried out. The optimum conditions for Mg(II) adsorption using 0.3 g coal bottom ash are at pH 5 with 60 minute contact. The Adsorption kinetics follow Ho model pseudo-second order with the rate constant 0.6182 and 0.998 correlation coefficient. These results highlight the potential of activated coal bottom ash as a low-cost, effective adsorbent for water treatment applications.
References
[1] Basuki, R., Apriliyanto, Y., Stiawan, E., Pradipta, A.R., Rusdiarso, B., & Putra, B.R. 2025. Magnetic hybrid chitin-horse manure humic acid for optimized Cd(II) and Pb(II) adsorption from aquatic environment. Case Stud. Chem. Environ. Eng. 11. 101138. https://doi.org/10.1016/j.cscee.2025.101138
[2] Dong, Y., Zhou, M., Xiang, Y., Wan, S., Li, H., & Hou, H. 2019. Barrier effect of coal bottom ash-based geopolymers on soil contaminated by heavy metals. RSC Advances. 9(42). 28695–28703. https://doi.org/10.1039/c9ra05542h
[3] Fitriana, D., Mudasir, M., Siswanta, D., 2020. Adsorption of Pb(II) from aqueous solutions on dithizone-immobilized coal fly ash. Key Eng. Mater. https://doi.org/10.4028/www.scientific.net/kem.840.57
[4] Huang, J., Yuan, F., Zeng, G., Li, X., Gu, Y., Shi, L., Liu, W., Shi, Y., 2017. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere. 173. 199-206. https://doi.org/10.1016/j.chemosphere.2016.12.137
[5] Huda, B.N., Wahyuni, E.T., Mudasir, M., 2021. Eco-friendly immobilization of dithizone on coal bottom ash for the adsorption of lead(II) ion from water. Results Eng. 10. 2021. https://doi.org/10.1016/j.rineng.2021.100221
[6] Irawan, C., Dahlan, B., & Retno, N. 2021. The effect of adsorbent mass, contact time and adsorbent activation using HCl on the effectiveness of heavy metal (Fe) reduction using fly ash as an adsorbent. Jurnal Teknologi Terpadu. 3(2). 89–96. https://doi.org/10.32487/jtt.v3i2.89
[7] Krisdiyanto, D., Khamidinal, & Faqih, A. 2022. Adsorption Cd(II) by zeolite from bottom ash modified by dithizone. Journal of Tropical Chemistry Research and Education. 4(2). 110–125. https://doi.org/10.14421/jtcre.2022.42-06
[8] Mudasir, M., Karelius, K., Aprilita, N.H., Wahyuni, E.T., 2016. Adsorption of mercury(II) on dithizone-immobilized natural zeolite. J. Environ. Chem. Eng. 4, 1839–1849. https://doi.org/10.1016/j.jece.2016.03.016
[9] Rahayu, I., Nazriati, Fajaroh, F. & Nur, A. 2019. Adsorpsi ion kadmium menggunakan silika xerogel berbasis abu bagasse. Journal Cis-Trans. 3(1). 10-16. http://dx.doi.org/10.17977/um0260v3i12019p010
[10] Rutskoy, B., Ozerov, G., & Bezrukov, D. (2024). The role of bond functions in describing intermolecular electron correlation for Van der Waals dimers: a study of (CH₄)₂ and Ne₂. Int. J. Mol. Sci. 25(3). 1472. https://doi.org/10.3390/ijms25031472
[11] Santosa, S.J., Narsito, & Lesbani, A. 2020. Sorption-desorption mechanism of Zn(II) and Cd(II) on Chitin. Indones. J. Chem. 20(3). 267–274. https://doi.org/10.22146/ijc.21772
[12] Shi, Q., Terracciano, A., Zhao, Y., Wei, C., Christodoulatos, C., Meng, X. 2019. Evaluation of metal oxides and activated carbon for lead removal: kinetics, isotherms, column tests, and the role of co-existing ions. Sci. Total Environ. 648, 176–183. https://doi.org/10.1016/j.scitotenv.2018.08.013
[13] Tajudin, W.S., Sunarti, S., & Manuhutu, J. B. (2023). optimasi massa adsorben dan Ph pada adsorpsi ion Fe menggunakan abu cangkang kelapa sawit. Molluca Journal of Chemistry Education. 13(2). 74–86. https://doi.org/10.30598/MJoCEvol13iss2pp74-86
[14] Vajargah, M.F. 2021. A Review on the effects of heavy metals on aquatic animals. Journal of Biomedical Research & Environmental Sciences. 2(9). 865-869. http://dx.doi.org/10.37871/jbres1324
[15] World Health Organization (WHO). 2017. Guidelines for Drinking-water Quality: Fourth Edition Incorporating the First Addendum. Geneva: World Health Organization.
[16] Yahya, M.D., Abubakar, H., Obayomi, K.S., Iyaka, Y.A., & Suleiman, B., 2020. Simultaneous and continuous biosorption of Cr and Cu (II) ions from industrial tannery effluent using almond shell in a fixed bed column. Results Eng. 6. 100113. https://doi.org/10.1016/j.rineng.2020.10011
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Sorption Studies

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









